Hypertension has been shown to be an independent, quantitative and reversible risk factor for CVD, including stroke, CHD, peripheral artery disease and cardiac failure, in primary and secondary prevention studies(Reference He and MacGregor1). Relatively small blood pressure (BP) reductions of about 5 mmHg in systolic BP can reduce the risk of strokes by one-third and coronary events by one-fifth(Reference MacMahon, Peto and Cutler2). The potential of both n-3 PUFA and B-vitamins in reducing BP was hypothesised several years ago(Reference Forman, Rimm and Stampfer3, Reference Knapp4); yet, no clear and definitive answer has been found in primary and secondary prevention trials(Reference Geleijnse, Giltay and Grobbee5–Reference van Dijk, Rauwerda and Steyn9).
A lowering effect of n-3 fatty acids on BP has been described in the literature, but the results are quite heterogeneous(Reference Geleijnse, Giltay and Grobbee5, Reference Hooper, Thompson and Harrison6). In addition, most studies used high doses of n-3 fatty acids(Reference Appel, Miller and Seidler10). n-3 Fatty acids can influence BP through various mechanisms including an interaction with angiotensin II. Indeed, n-3 PUFA reduce angiotensin-converting enzyme activity as well as angiotensin II formation, resulting in an improved vasodilation and arterial compliance of both small and large arteries(Reference Cicero, Ertek and Borghi11). Other mechanisms of the action proposed are reduced production of the vasoconstrictor thromboxane A2, increased synthesis of NO and an effect on autonomic nerve function(Reference Mori and Woodman12).
With regard to B-vitamins, and particularly folic acid, a lowering effect on BP has been described extensively in the literature(Reference McRae7, Reference van Dijk, Rauwerda and Steyn9, Reference Cagnacci, Cannoletta and Volpe13–Reference Williams, Kingwell and Burke16). The effect of folic acid on BP could be due to its action on homocysteine. Plasma homocysteine levels have been positively associated with BP in cross-sectional studies(Reference Nygard, Vollset and Refsum17, Reference Kahleova, Palyzova and Zvara18). Furthermore, in several intervention studies, supplementation with folic acid or 5-methyltetrahydrofolate decreased homocysteine concentration(19).
Although the role of B-vitamins and n-3 fatty acids on BP is mechanistically grounded, this has not been consistently shown in intervention studies, which have usually been of short-to-medium duration and have generally employed supplements at high doses.
The Supplementation in Folate (and B-vitamins) or n-3 (Supplémentation en Folates et Omega-3; SU.FOL.OM3) trial(Reference Galan, Kesse-Guyot and Czernichow20) provides a unique opportunity to assess in a large sample of patients with a history of CVD, the impact of supplementation with B-vitamins (5-methyl-tetrahydrofolate, vitamin B12 and vitamin B6) and/or n-3 PUFA for 5 years on BP. The primary objective of the SU.FOL.OM3 trial was to examine the effect of these interventions on the recurrence of CVD outcomes. We conducted post hoc analyses in the SU.FOL.OM3 cohort to study the effect of supplementation with B-vitamins and/or n-3 fatty acids for a long duration (5 years) on BP in a population with CVD.
Subjects and methods
Study design
The SU.FOL.OM3 trial is a multi-centre, double-blind randomised trial with a placebo-controlled factorial design that has evaluated the separate and combined effects of daily supplementation with B-vitamins and n-3 PUFA on the prevention of CVD(Reference Galan, Kesse-Guyot and Czernichow20). The detailed protocol has been described previously(Reference Galan, Kesse-Guyot and Czernichow20). Briefly, patients with CVD (myocardial infarction, stroke and unstable angina) were randomly assigned in a 2 × 2 factorial design to one of four groups: B-vitamins (5-methyl-THF (560 μg); vitamin B6 (3 mg) and vitamin B12 (20 μg)) and a placebo capsule for fatty acids; n-3 fatty acids (600 mg of EPA and DHA at a ratio of 2:1 in the form of TAG) and a placebo capsule for B-vitamins; both B-vitamins and n-3 fatty acids; or placebo capsules for both treatments. The patients took two capsules daily in a double-blind manner for 5 years.
B-vitamins placebo and n-3 fatty acids placebo capsules were developed to maintain the blindness of the trial, and were manufactured to be indistinguishable from their corresponding treatment capsule by external colour, size or taste (use of fish oil flavour for n-3 placebo). The supplements were provided by Merck Eprova AG (Schaffhausen, Switzerland) (for 5-methyl-THF), Roche Laboratories (Basel, Switzerland) (for vitamin B6 and vitamin B12) and Pierre Fabre (Ramonville, France) (for n-3 PUFA). The gelatine placebo capsules contained liquid paraffin, and for the n-3 placebo the capsules additionally contained fish oil flavour. The gelatine capsules were manufactured by Catalent Pharma Solutions (Beinheim, France; Swindon, UK).
Randomisation was performed in blocks using a computer-based randomisation programme and stratified by three age categories (44–54, 55–64 and 65–80 years), sex, inclusion criteria for CVD and recruitment centre. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects were approved by the ethics committee ‘Comité Consultatif pour la Protection des Personnes se prêtant à la Recherche Biomédicale’ (CCPPRB no. 1933) of Paris-Cochin, and the data protection board ‘Comité National Informatique et Liberté’ (CNIL no. 901230). Written informed consent was obtained from all patients. All the analyses and interpretation were carried out independent of the organisations that funded the study. The present trial was registered under the no. ISRCTN41926726 in Current Controlled Trials (http://www.controlled-trials.com/ISRCTN41926726).
Study population
Participants with a history of CVD were recruited via a network of 417 cardiologists, neurologists or other physicians throughout France. Men and women aged 45–80 years who had a history of an acute coronary or cerebral ischaemic event occurring within 1–12 months before randomisation were eligible to participate.
Post hoc analyses were performed for all the patients who were included in the primary analyses for the SU.FOL.OM3 study(Reference Galan, Kesse-Guyot and Czernichow20). Among a total of 2501 participants (1987 men and 514 women), the 2 × 2 random assignment yielded four groups: B-vitamins and placebo for n-3 fatty acids (n 622); n-3 fatty acids and placebo for B-vitamins (n 633); both active treatments (n 620); and placebo for both treatments (n 626). Among the 2501 participants, 2170, 2066, 1924, 1618 and 1184 individuals attended the non-mandatory annual examinations at years 1–5, respectively. The median follow-up time was 4·7 years.
Measurements
BP was measured at baseline and during annual examinations for the subsequent 5 years. BP was recorded by trained study staff for each patient in the sitting position, after a 5 min rest, with a semi-automatic device (Digital blood pressure monitor OMROM UA-787; OMRON Corporation, Kyoto, Japan) using standardised protocols. We took two measurements. In the case of a BP difference greater than 10 mmHg for either systolic BP or diastolic BP, BP was measured again after a 5 min rest. The last two BP values were averaged.
BMI (kg/m2) was calculated at each annual examination from the corresponding measurements of height and weight recorded by the trained staff following standard protocols. Data on age, level of education (elementary school, secondary school and university or equivalent), smoking status (current smokers, former smokers and non-smokers) and medication use were collected at inclusion via questionnaires.
Blood samples were obtained at baseline and at each annual follow-up examination after a 12 h fast. All biochemical measurements were centralised. Total cholesterol, TAG, and HDL- and LDL-cholesterol were measured by the enzymatic method with the use of the C8000 System (Abbott, Rungis, France); plasma homocysteine, serum folate and vitamin B12 were measured by a competitive immunoassay with direct chemiluminescence detection; and pyridoxal 5′-phosphate (circulating form of vitamin B6) was determined in the plasma by HPLC.
Plasma LDL-cholesterol >2·55 mmol/l, plasma homocysteine >15 μmol/l and BMI >25 kg/m2 were used to define hypercholesterolaemia, hyperhomocysteinaemia and overweight, respectively. Independently of anti-hypertensive drug prescription, hypertension was considered to be present whenever the achieved systolic and the diastolic BP were higher than 140 and 90 mmHg, respectively.
Compliance was assessed by means of self-reported questionnaires at 6-month intervals (or by a telephone interview by study physicians). The patients were considered compliant if they took at least 80 % of their allocated treatment. In addition, change in plasma B-vitamins (n 2147) was also examined. Fatty acid composition of plasma lipids was determined on a subgroup (n 682) as well, at baseline and 1 year post-intervention, by GC of the corresponding methyl esters.
Statistical analysis
To examine the effect of the intervention, mixed linear models with repeated measures were built using the difference in BP between two successive examinations as the dependent variable. This model was repeated with adjustment for subject characteristics at baseline (inclusion criteria for CVD, sex, level of education and age), and clinical (BP, number of anti-hypertensive drugs and BMI) and biological (plasma homocysteine and cholesterol concentrations) data assessed at the beginning of each period, as well as change in clinical and biological variables between two successive measurements. All two-by-two interactions between independent variables were tested in these mixed models. Bonferroni's correction for multiple testing was applied. None of the interactions were significant.
To illustrate the effect of supplements (B-vitamins or n-3 fatty acids) on the evaluation of BP, we first obtained combined n-3 or combined B-vitamins groups(Reference Galan, Kesse-Guyot and Czernichow20) by merging the groups receiving the n-3 supplement (either alone or with B-vitamins) and the groups receiving B-vitamins (either alone or with n-3 fatty acids), respectively. The mixed linear model with repeated measures was run with the combined n-3 group and the combined B-vitamins groups and interaction terms (n-3 × time, B-vitamins × time, n-3 × B-vitamins and n-3 × B-vitamins × time). BP at successive measurements was defined as the dependent variable. Interaction of intervention group × time with the inclusion criteria for CVD, sex, age, overweight status, hyperhomocysteinaemia and BP at baseline was tested and found non-significant.
All statistical analyses were performed using SAS (SAS Institute, Inc., Cary, NC, USA) version 9.1 software.
Results
Patient characteristics at baseline are shown for all four supplementation groups in Table 1. The participants were 61·3 (sd 9·0) years old and most were male (79·5 %). Among the three inclusion criteria, myocardial infarction was the most common reason for inclusion. At inclusion, 979 participants had a high achieved BP (39·2 %); average systolic BP was 133·4 (sd 21·4) and diastolic BP was 83·2 (sd 12·3.) Of the participants, 74 % were overweight; average BMI was 27·6 (sd 4·0); 53 % had hypercholesterolaemia and 29·3 % had hyperhomocysteinaemia. The participants with a high achieved BP at baseline were older, had higher BMI and had higher plasma total cholesterol and homocysteine concentrations than individuals with controlled BP (P < 0·0001).
Table 1 Baseline characteristics of participants
Mean values, standard deviations, number of participants and percentages

Compliance with the supplementation regimen was high. The overall response rate for return of completed questionnaires was 99, 96, 94 and 95 % at 6, 12 and 24 months and at the end of the trial, respectively; 86 % of the respondents were considered compliant with the study medication (took >80 % of assigned capsules) and compliance was similar (approximately 86 %) in all four treatment groups.
As reported previously(Reference Galan, Kesse-Guyot and Czernichow20), the median (interquartile range) baseline concentration for plasma folate was 6·7 (5·2–8·7) ng/ml, vitamin B12 was 365 (296–463) pg/ml and homocysteine was 12·8 (10·8–15·8) μmol/l; EPA represented 1·16 (0·81–1·73) % of total fatty acids and DHA represented 2·58 (2·05–3·28) % of total fatty acids at baseline examination. Baseline plasma concentrations for B-vitamins, homocysteine and n-3 fatty acids were not statistically different among the four groups. Allocation to B-vitamins was associated with a 20 % (2·5 μmol/l) reduction in median plasma homocysteine concentration after 1 year and 19 % (2·7 μmol/l) reduction at the end of the trial compared with placebo. Allocation to B-vitamins was associated with a 146 % increase in serum folate, 35 % increase in serum vitamin B12 and 116 % increase in serum vitamin B6. Supplementation with n-3 fatty acids was associated with a significant increase in median plasma EPA (by 79 %) and in median DHA (by 18 %) compared with the placebo at 1 year of intervention(Reference Galan, Kesse-Guyot and Czernichow20).
No intervention effect was observed with either B-vitamins, n-3 fatty acids or their combination on change in systolic BP, diastolic BP, mean BP or pulse BP in crude and adjusted models (Table 2). BP change was not associated with change in homocysteine concentration in any of the groups in all the regression models examined (P>0·10; data not shown).
Table 2 Effect of supplementation with n-3 fatty acids or B-vitamins on blood pressure (BP) measurements

Fig. 1 illustrates the evolution of BP over the study period for combined n-3 fatty acids groups v. placebo (for n-3 fatty acids; Fig. 1(a)) and for the combined B-vitamins groups v. placebo (for B-vitamins; Fig. 1(b)). There was no significant effect of supplementation with either n-3 fatty acids or B-vitamins on systolic BP, diastolic BP, mean BP and pulse BP.
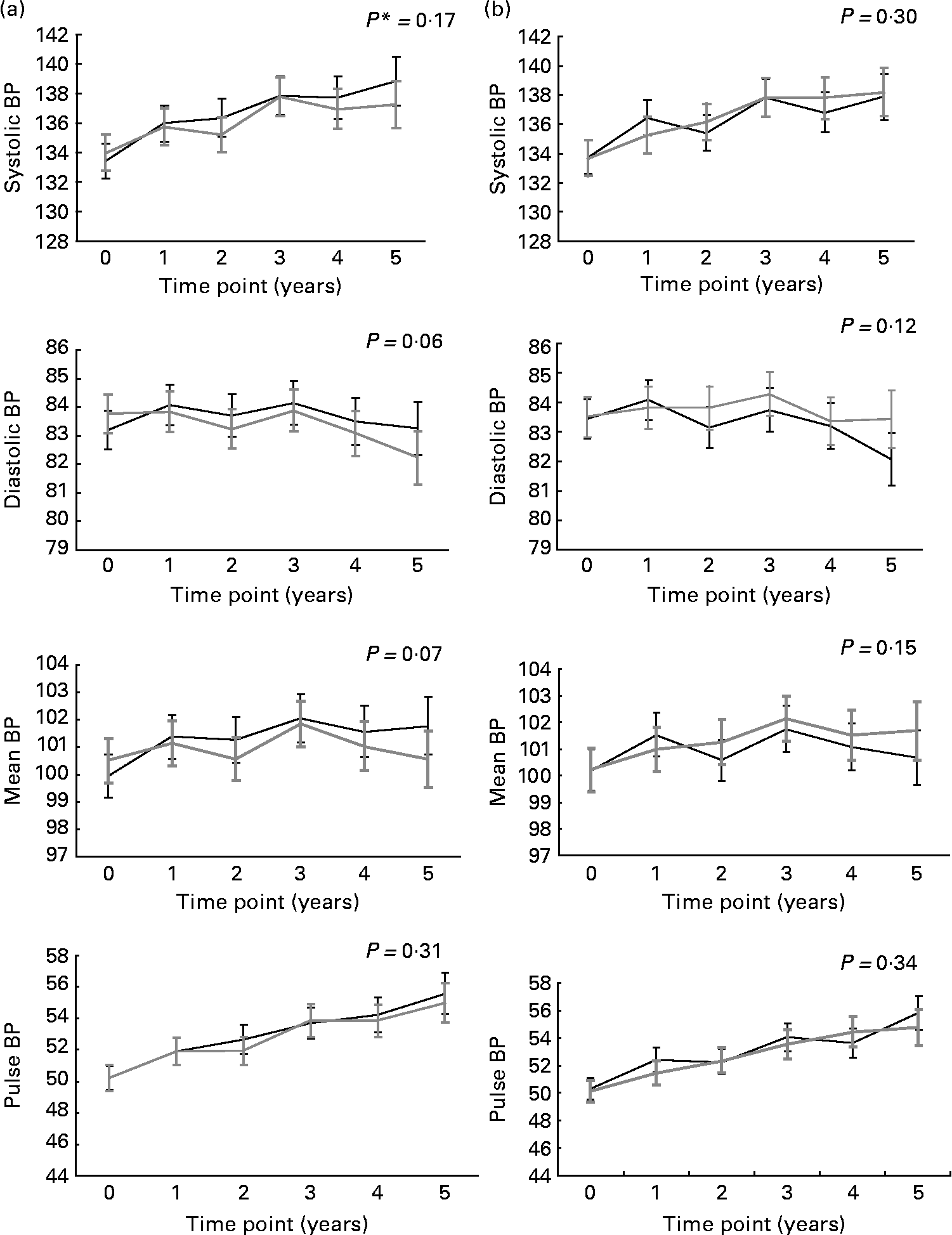
Fig. 1 Evolution of systolic, diastolic, mean and pulse blood pressure (BP) in the n-3 (![]() )- and (b) B-vitamins (
)- and (b) B-vitamins (![]() )-supplemented groups and the corresponding placebos (
)-supplemented groups and the corresponding placebos (![]() ) over the 5 years follow-up. Values are means with 95 % CI represented by vertical bars. * P for trend obtained with t statistic from the mixed linear models.
) over the 5 years follow-up. Values are means with 95 % CI represented by vertical bars. * P for trend obtained with t statistic from the mixed linear models.
Discussion
In this secondary prevention trial with patients with established CVD, no effect on BP of supplementation for 5 years with either n-3 PUFA or B-vitamins was found. This result is consistent with the main analysis of the trial not showing any effect of the supplementation on the recurrence of cardiovascular or neurovascular events(Reference Galan, Kesse-Guyot and Czernichow20). This finding was unchanged when analyses were restricted to individuals with hypertension at baseline.
Supplementation with B-vitamins significantly reduced homocysteine concentrations. The change in BP between successive examinations was not associated with change in homocysteine concentration in any of the supplementation groups.
Several studies have investigated the relationship between n-3 fatty acid supplementation and BP and found heterogeneous results(Reference Geleijnse, Giltay and Grobbee5, Reference Hooper, Thompson and Harrison6, Reference Appel, Miller and Seidler10, Reference Cicero, Ertek and Borghi11, Reference Morris, Sacks and Rosner21, Reference Tavazzi, Maggioni and Marchioli22). In a meta-analysis of thirty-six trials, Geleijnse et al. (Reference Geleijnse, Giltay and Grobbee5) reported a reduction in both systolic and diastolic BP due to supplementation in n-3 fatty acids. Previous meta-analyses(Reference Appel, Miller and Seidler10, Reference Morris, Sacks and Rosner21) also found that supplementation with fish oil slightly lowered BP. However, most studies in these meta-analyses used relatively high doses of n-3 fatty acids and were short in duration (most lasted for < 3 months). A more recent review from the Cochrane Collaboration(Reference Hooper, Thompson and Harrison6) based on the results from seven studies (2743 participants in total), with a duration of at least 6 months, did not show any effect of n-3 supplementation on either systolic or diastolic BP. This non-significant result was confirmed after 4 years of supplementation (1 g/d of n-3 fatty acids) in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico–Heart Failure trial (patients with previous heart failure, n 6975)(Reference Tavazzi, Maggioni and Marchioli22).
We did not find any BP-lowering effect attributable to n-3 fatty acids at dietary doses in the SU.FOL.OM3 study. Apart from a true absence of effect, several hypotheses could explain this finding. First, the doses used in the present study were dietary doses and thus relatively small. However, the plasma concentrations of EPA and DHA obtained after supplementation with n-3 fatty acids after 1 year of intervention were similar to those observed customarily in high fish consumers in France, i.e. those in the highest quartile of fish consumption in a national sample in the SU.VI.MAX study conducted in our laboratory (S Hereberg and P Galan, unpublished results), where fatty acids were determined in the same manner as in the present study. We could not compare these concentrations with those described in the literature due to differences in the populations examined(Reference Ayer, Harmer and Xuan23, Reference Mayneris-Perxachs, Bondia-Pons and Serra-Majem24), type and dose of supplementation(Reference Ishikawa, Yokoyama and Saito25), as well as variability in the analytical methods for fatty acids. It may be speculated that even higher circulating levels need to be achieved in order to observe protective effects from a secondary prevention perspective. Second, the description of a positive effect in short-term trials(Reference Geleijnse, Giltay and Grobbee5, Reference Appel, Miller and Seidler10, Reference Morris, Sacks and Rosner21) could also indicate that the BP-lowering effect of n-3 fatty acids is more pronounced in the short term (less than a few months). Due to the lack of literature on supplementation with n-3 fatty acids for >4 years, whether a longer-term effect with higher doses of n-3 fatty acids could be seen cannot be excluded. Finally, the vasodilative properties of n-3 fatty acids could be inefficient on arteries already degraded by atherosclerosis, explaining their lack of an anti-hypertensive effect in secondary prevention.
With regard to the potential effect of B-vitamins, and particularly folate, several studies and meta-analyses have reported a positive effect on BP reduction in both primary and secondary prevention trials lasting between a few weeks and a few years(Reference McRae7, Reference van Dijk, Rauwerda and Steyn9, Reference Cagnacci, Cannoletta and Volpe13, Reference Papandreou, Malindretos and Arvanitidou15). However, we did not observe any relationship between B-vitamins supplementation at dietary doses, which are much smaller than those used in previous studies, and a change in BP. The present findings suggest that dietary doses of B-vitamins are insufficient to induce a reduction in BP; the dose–effect relationship needs further examination in future studies.
We also studied whether a change in the homocysteine level, possibly induced by folate, was associated with BP change. Much evidence suggests that supplementation with folic acid or folate can lower homocysteine levels(19). Furthermore, a positive association between plasma homocysteine levels and BP or hypertension has been described in some cross-sectional studies(Reference van Dijk, Rauwerda and Steyn9, Reference Nygard, Vollset and Refsum17, Reference Kahleova, Palyzova and Zvara18, Reference Budge, de Jager and Hogervorst26–Reference Sutton-Tyrrell, Bostom and Selhub29); even though others have reported opposite results(Reference Dinavahi, Cossrow and Kushner30–Reference Sundstrom, Sullivan and D'Agostino32). Certain primary and secondary intervention trials, varying from a few weeks to 2 years, have also described the BP-lowering effect with folic acid supplementation, which was associated with a decrease in homocysteine levels(Reference McRae7, Reference van Dijk, Rauwerda and Steyn9, Reference Cagnacci, Cannoletta and Volpe13–Reference Williams, Kingwell and Burke16); this effect seems to occur only with high doses of folate or folic acid (>5 mg/d). In contrast, consistent with the present findings, others have reported no effect of supplementation with B-vitamins (including folic acid/folate) on BP. In an intervention trial lasting 2 years involving 276 healthy older adults with hyperhomocysteinaemia, who took either a daily supplement of B-vitamins (folate (1 mg), vitamin B12 (500 μg) and vitamin B6 (10 mg)) or a placebo(Reference McMahon, Skeaff and Williams33), no effect on BP was observed. Similarly, in the large Vitamin Intervention for Stroke Prevention (VISP) trial (including 3361 individuals with a prior history of a stroke) using B-vitamins at either a low dose (200 μg pyridoxine, 6 μg cobalamin and 20 μg folic acid) or a high dose (25 mg pyridoxine, 0·4 mg cobalamin and 2·5 mg folic acid), BP did not change following 2 years of supplementation(Reference Toole, Malinow and Chambless8). Taken together, the mixed findings in the literature suggest that the lack of a beneficial effect on BP observed in the present study is most probably related to the low dose used. The lack of association between change in homocysteine and change in BP in the present study could be related to arterial stiffness in the study sample involving patients with established CVD. It has been shown that in hypertensive patients, the levels of homocysteine are strongly and independently correlated to arterial stiffness(Reference Blacher, Demuth and Guerin34, Reference Bortolotto, Safar and Billaud35). Plasma homocysteine and aortic pulse wave velocity are higher in patients presenting with clinical vascular disease. It is possible that the effect of a change in homocysteine concentrations on reduction in BP is modified by the degree of arterial stiffness and this needs further examination.
Certain limitations of the study must be considered. The study involved low doses of supplements; thus it cannot answer whether an effect of B-vitamins or n-3 fatty acids would be observed at higher doses in long-term trials. The study was conducted in a double-blind manner, which should have avoided a systematic bias induced by change in diet and/or supplementation practices of the participants, but this cannot be ruled out. In order to address this, we examined the evolution of the plasma concentrations of B-vitamins and n-3 fatty acids in the study. The groups did not differ on either B-vitamins or n-3 fatty acids at baseline, and significant increases were observed in B-vitamins and n-3 fatty acids in the groups receiving the corresponding supplements and not in any other groups(Reference Galan, Kesse-Guyot and Czernichow20). Thus dietary changes, if any, did not confound the intervention design.
The present study has several strengths. First, to our knowledge, the present intervention trial is one of the few that had a long duration (median 4·7 years). Second, its aim was to test the benefit of low doses of supplements that could be achieved via dietary means. The study design involving a double-blind, randomised controlled trial is another strength. Thus, the findings of a lack of benefit of low-dose supplementation with B-vitamins or n-3 fatty acids in a population with established CVD are novel and deserve confirmation in other populations.
In summary, the present results do not support any effect of supplementation with B-vitamins or n-3 fatty acids at dietary doses on BP in individuals with a prior history of CVD.
Acknowledgements
We thank the health professionals, staff at the coordinating centre and study centres as well as the patients who participated in the SU.FOL.OM3 trial. We also acknowledge Gwenael Monot for data management. The SU.FOL.OM3 study was funded by the French Ministry of Research (grant R02010JJ), the Ministry of Health (Direction Générale de la Santé; DGS), Sodexo, Candia, Unilever, Danone, Roche Laboratories, Merck EPROVA GS and Pierre Fabre Laboratories. None of the funding organisations had any involvement in the design and conduct of the study, or in the collection, management, analysis and interpretation of the data, or in the preparation, review and approval of the manuscript. None of the authors has any competing interests, and all are independent of the funding bodies. P. G. and S. H. designed the study; P. G., S. H. and J. B. conducted the study; F. S. d. E. analysed the data and wrote the manuscript with N. A.; A.-C. V., C. J. and N. A. assisted with the data analysis; F. S. d. E. had primary responsibility for the final content. All authors contributed to the manuscript development, and read and approved the final manuscript.







