Abstract
Nanoparticle contaminants enter aquatic ecosystems and are transported along the stream network. Here, we demonstrate a novel pathway for the return of nanoparticles from aquatic to terrestrial ecosystems via cross-boundary subsidies. During their emergence, trichopteran caddisflies carried titanium dioxide and gold nanoparticles into their terrestrial life stages. Moreover, their emergence was delayed by ≤30 days, and their energy reserves were depleted by ≤25%. Based on worst case estimates, it is suggested that terrestrial predators, such as bats feeding on aquatic prey, may ingest up to three orders of magnitude higher gold levels than anticipated for humans. Additionally, terrestrial predator species may suffer from alterations in the temporal availability and nutritional quality of their prey. Considering the substantial transfer of insect biomass to terrestrial ecosystems, nanoparticles may decouple aquatic and terrestrial food webs with important (meta-)ecosystem level consequences.
Similar content being viewed by others
Introduction
Over 1,000 products, including sunscreens, textiles, and self-cleaning surfaces, contain engineered nanoparticles or are produced utilizing nanotechnology. As a result, the unintentional introduction of engineered nanoparticles into freshwater environments, mainly via wastewater treatment plant effluents1, is increasing2 and represents a significant share of the manufacturing production volume3. Nanoparticles are transported downstream until their ultimate release into the marine ecosystem. However, the potential for nanoparticle transport from the aquatic environment back to terrestrial ecosystems has been neglected thus far. In this context, the transfer of nanoparticles via merolimnic aquatic insects represents a possible pathway directly into terrestrial food webs. Because nanoparticles are consumed within the insect aquatic life stages, this process likely results in a higher nanoparticle bioavailability for terrestrial species than if they were associated with the soil matrix. Although studies have shown that nanoparticles can be transmitted along the aquatic4,5 and terrestrial6 food chains, cross-ecosystem dietary exposure has not yet been examined. Additionally, it is unknown whether the temporal availability and quality of these subsidies, namely the emergence pattern and nutritional value (e.g., energy reserves) of aquatic insects, are affected by nanoparticles. Both the trophic transfer of nanoparticles via aquatic subsidies and changes in the availability and quality of this subsidy may be of serious concern for the well-being of terrestrial predators and their offspring, including endangered species of amphibians, lizards, birds, bats and spiders7,8,9. Indeed, a contamination of aquatic systems by chemicals of anthropogenic origin such as metals, pharmaceuticals and others suggest alterations in the aquatic-terrestrial coupling10,11,12,13,14,15,16 warranting further studies17.
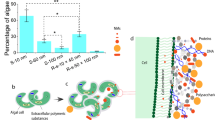
To investigate the transfer of nanoparticles from an aquatic to a terrestrial ecosystem, this study employed laboratory flow-through stream microcosms (n = 24; Fig. 1a) over a period of 140 days. In this system, the transfer of 62.3-nm titanium dioxide (4 and 400 µg nTiO2/L; Fig. 1b) and 15.1-nm gold (6.5 µg nAu/L; Fig. 1c, see also Table S3) nanoparticles via the caddisfly Chaetopteryx villosa developing from aquatic larvae into a terrestrial adult stage was investigated. Nanoparticles were assumed to be taken up by C. villosa from the water phase or from consumed leaf material that adsorb nanoparticles during exposure (Table S1). In parallel, the temporal emergence pattern and the energy reserves (i.e., lipid stores) of this species, which can be found in large quantities in slow flowing streams18, were monitored. In addition, the photocatalytic effects of nanoparticles on the responses of Chaetopteryx were assessed by exposure to ultraviolet (UV) irradiation at ambient intensities UV-A: 9.7 W/m2, UV-B: 0.35 W/m2 19, in the presence and absence of 4 or 400 µg nTiO2/L20.
Experimental set-up. (a) 24 stainless steel streams (N = 24; volume: 40 L each) simulated freshwater stream habitats in the laboratory. Each stream was equipped with artificial sediment (i.e., glass spheres), leaves and caddisfly larvae. The units were run in flow-through mode, ensuring a constant concentration of nanoparticles over the 140 day duration of the study. (b) Transmission electron microscopy (TEM) image of nTiO2. (c) TEM image of nAu.
Results and Discussion
Although none of the treatments affected the endpoints overall leaf consumption, survival and emergence rate of caddisfly larvae relative to the controls (Table S2), the temporal emergence pattern (Fig. 2a; Fig. S1), the energy reserves of (Fig. 2b) and the concentration of nanoparticles in the adult organisms (Table 1) deviated considerably among the treatments. The presence of nAu, for example, delayed the emergence (defined as the study duration at which 50% of Chaetopteryx individuals have emerged) by up to one month (Fig. 2a). In addition, the energy reserves, measured as total lipid concentration in emerged adults, were reduced by up to 25% (Fig. 2b). UV-irradiation alone delayed emergence by eleven days; in combination with 4 and 400 µg nTiO2/L, this effect was significantly extended to 16 and 20 days, respectively, presumably due to the formation of reactive oxygen species21. Moreover, lipid concentrations of these adults were significantly decreased (Fig. 2b). Because the lower experimental concentration of nTiO2 (i.e., 4 µg/L) is at least seven times less than nTiO2 concentrations measured in a recreational lake in central Europe and wastewater treatment plant effluents22,23, the delay in emergence caused by the combination of nTiO2 and ambient UV-irradiation can (despite the fact that the study was performed under controlled environmental conditions) be considered environmentally relevant with potentially strong implications for aquatic and terrestrial ecosystems. As outlined above, nAu exposure resulted in an even more severe delay in emergence and depletion of energy reserves at a concentration considered to be environmentally safe for Au ions – not nanoparticles, which are usually deemed to be less toxic – based on European regulations24. Although the concentration assessed in the present study is an order of magnitude above the nAu concentrations currently predicted for aquatic ecosystems25, the data presented here raise concerns for unanticipated effects at the ecosystem level. This concern is underpinned by the substantial effect that shifts in the temporal emergence pattern and the nutritional value (i.e., energy reserves) of emerging adult insects may have on terrestrial predators, such as spiders and adult amphibians, lizards, bats and birds26, that may ultimately influence their reproductive success27,28. In particular, migratory bird species must fill their energy reserves prior to or following seasonal movements, and changes in the temporal emergence pattern and lipid concentrations of merolimnic prey insects may have serious implications for the birds’ life cycles8.
Effects of nanoparticles on caddisflies. Emergence pattern (a) and mean lipid concentrations (b) in adult caddisflies exposed as larvae for 140 days in stream microcosms to nanoparticles. A and B above the error bars refer to a statistically significant difference relative to the control or the UV treatment, respectively.
Most importantly, emerging aquatic insects carried substantially elevated concentrations of either TiO2 or Au following exposure to nanoparticles (Table 1). Environmental scanning electron microscopy coupled to energy dispersive X-ray spectroscopy images of thin image sections support the uptake of nanoparticles into abdominal tissue of the adult insects (Fig. 3). A potential adhesion of nanoparticles from the medium to the outer surface of the adults following pupation, as simulated by submersing control flies into the respective media, does not account for the measured TiO2 and Au concentrations in the body (Table S1). Our finding demonstrates the potential for nanoparticles to be transferred from the aquatic ecosystem into the terrestrial food web – a novel pathway for nanoparticles in the environment. In the gut of a terrestrial predator, nanoparticles may affect the digestive system29 and thus the physiological constitution. Moreover, we calculated theoretical intake rates based on the estimated daily consumption of approximately 6 g of insects during pregnancy and lactation of little brown bats (Myotis lucifugus)30 assuming that their Au concentration equals the median concentrations detected in adults of the present study. These extrapolations suggest a theoretical ingestion of nAu with the aquatic prey at levels that are a factor of 1000 above those considered as maximum intake for humans as a consequence of gold being used as food additives31. At the same time, it was indicated that dietary ingestion of gold at levels comparable to this maximum intake can cause skin rash in humans32. Although these insights are worrying for aquatic-terrestrial meta ecosystems17, predicting the implications of this exposure for terrestrial food webs remains difficult33, particularly as the present study followed a relatively simplistic experimental setting involving one species under relatively well controlled environmental conditions. Nonetheless, based on simplistic assumptions that (i) the concentration of nAu and nTiO2 is comparable among merolimnic insect species and (ii) environmental concentrations reach the levels tested here, nAu and nTiO2 fluxes from the aquatic to the terrestrial environment can be as high as 1.4–31.8 mg and 2.4–56.5 mg each per square meter of water surface per year, respectively. This estimate is based on the median nanoparticle concentrations measured in the present study and the total annual biomass of emerging merolimnic insects from streams as reviewed by Raitif et al.34. These data therefore may raise concerns about the consequences for higher trophic levels in riparian systems, including endangered species, associated with the transfer of contaminants such as nanoparticles from the aquatic back to the terrestrial food web. Although recent explorations of the movement and impact of contaminants, such as microplastics10, metals11,12,13,14, polychlorinated biphenyls15, pharmaceuticals16 and in the present study nanoparticles, highlight impairments in the aquatic-to-terrestrial subsidy, research addressing this question is still in its infancy warranting further studies17.
Nanoparticles in adult caddisflies. (a) Environmental scanning electron microscopy (ESEM) images, diagrams of the energy dispersive X-ray spectroscopy confirming that the particles are Au can be found in Fig. S2 and (b), ESEM image of nTiO2 in adult caddisflies. Arrows point to nanoparticles in the organisms’ abdominal tissue.
Materials and Methods
Nanoparticles
nTiO2 and nAu have been selected as model nanoparticles for the following reasons: Titanium dioxide is one of the most frequently applied nanoparticles worldwide used in personal care products, coatings, paints, and pigments35,36, entering the natural environment for instance via wastewater treatment plant effluents and landfill leachates. Gold, in contrast, has a low background concentration in environmental matrices, increasing the sensitivity of the chemical analyses and thus the possibility to quantify any potential transfer of nAu across ecosystem boundaries as part of the emerging insects.
The titanium dioxide product used in this study was purchased as a powder from Evonik (P 25; Germany) featuring an advertised primary particle size of 21 nm and crystalline composition of approximately 70% of anatase and 30% of rutile. Its advertised surface area was approximately 50 m2/g. The powder was transferred into a dispersant additive-free, size-homogenized, charge-stabilized suspension by stirred media milling (PML 2, Bühler AG, Switzerland37) at a concentration of 83.87 g nTiO2/L in deionized water.
Gold nanoparticles were synthesized by reducing Au3+ with tri-sodium citrate, essentially following the protocol published by McFarland et al.38. Briefly, a 1 mM hydrogen tetrachloraurate (HAuCl4*3H2O; Sigma-Aldrich, Germany) solution in sterile deionized water was stirred and heated until boiling. When the solution reached boiling, tri-sodium citrate (Na3C6H5O7; 38.8 mM; 15% of the volume of the diluted hydrogen tetrachloraurate solution) was quickly added. Heating and stirring were ceased after the solution turned a deep red color, resulting in a 79.95 mg nAu/L stock suspension. The particle size distributions of both stock suspensions were determined by dynamic light scattering (DLS; DelsaNano C, Beckman Coulter, Germany), and their development in the test medium (SAM-S5) over a 24 h period (the time of one water exchange) is described in Table S3.
Test organisms
The test species Chaetopteryx villosa (4th instar) was sampled from the Sauerbach, a near-natural stream negligibly impacted by settlement or agricultural activity (49°05′N; 7°37′E). In the laboratory, the animals were acclimatized to experimental conditions in stainless-steel artificial streams (120 × 30 × 20 cm; water volume 50 liters) filled with SAM-5S medium39 at 14 ± 1 °C for 14 days. Within each artificial stream microcosm, Chaetopteryx larvae (length greater than 10 mm) were kept in groups of 40 in stainless steel cages (70 × 14 × 20 cm) covered at the in-flow and out-flow with a 0.5 mm mesh screen. Each cage was equipped with artificial sediment comprised of 1.2 kg glass spheres of two size classes (1.0 and 0.2 kg with diameters of 105–210 µm and 800–1200 µm, respectively; Worf Glaskugeln GmbH, Germany). Glass spheres were used instead of natural sediment to minimize cross contamination with titanium from sources other than the water. Cages were deployed directly in front of the paddle wheel, which simulated running water conditions at a velocity of 0.1 m/s within the cages.
Experimental set-up
In total, the experiment involved 24 independent stainless-steel artificial streams, while each of the six independent technical control units (Pequitec, Switzerland) supplied four streams with the respective test medium (i.e., SAM-5S; water quality parameters are detailed in Table S4). Thus, a continuous medium exchange in the experimental units was ensured (one complete water exchange per 24 h), as well as dosing with the respective nanoparticles (see below). Dosing was initiated following the 14-day acclimatization phase. The artificial stream system was coupled with a light system simulating daylight and emitting UV-A and UV-B (Magic Sun 23/160 R 160 W, Heraeus, Germany) light at intensities of 9.7 and 0.35 W/m2, respectively, at the water surface (measured with a RM12 radiometer; Dr. Gröbel UV-Elektronic GmbH, Germany). These intensities are less than 25% of the peak UV-intensities measured during summer in Central Europe19 and represent values measured on a cloudy summer day near the test species sampling site40. Thus, the UV-intensities employed in the present study can be considered environmentally relevant, especially during the summer period. The day/night rhythm was set at 12/12 hours. Depending on the treatment, a UV-transparent or -filtering film (LLumar® UV CL-SR PS, SUNPOINT, Germany) covered the artificial stream. This film also served as a barrier to emerging insects.
The experimental system was used to establish six different treatments: (i) a control without the addition of nanoparticles or direct exposure to UV-irradiation, (ii) one treatment assessing the implication of UV-irradiation in the absence of nanoparticles, (iii) one treatment assessing for the implications of 4 µg nTiO2/L in the absence of UV, (iv) one treatment using the same concentration of nTiO2 in presence of UV, (v) one treatment investigating the combined effects of 400 µg nTiO2/L and UV-irradiation, and (vi) one treatment that evaluated the effect of 6.5 µg nAu/L in the absence of UV. Each of these treatments was replicated four times and assessed over the course of 140 days. During the study, caddisfly larvae received approximately 10 g (dry weight) of preconditioned black alder leaves see for details41 every other week, while all leaf material remaining from the former addition was removed, dried (at 60 °C for 48 h) and weighed to calculate the consumed leaf mass. Subsequently, the leaves were stored frozen for chemical analysis. At the same time, dead larvae were removed from the system. In addition, each artificial stream system was checked for emerging aquatic insects daily, which were frozen until further use.
Lipid analysis
The lipid content of 10–15 lyophilized and weighed (to the nearest 0.01 mg) adult insects per treatment was analyzed following the method described by Van Handel42: Lipids were extracted in a 1:1 chloroform:methanol (v:v) solution and reacted with sulfuric acid and vanillin-phosphoric acid reagent. For quantification, absorbance at 490 nm was measured and read against a standard curve prepared from commercially available soybean oil (Sojola Soja-Öl, Vandemoortele, Germany).
Chemical analysis
To monitor nanoparticle concentrations in the test medium, water samples were taken prior to the start and once a month during the experiment and were immediately analyzed using inductively coupled plasma quadrupole mass spectrometry ICP-MS, cp.43. Briefly, the ICP-MS (XSeriesII, Thermo Fisher Scientific, Germany) was equipped with a FAST autosampler (ESI, Thermo Fischer Scientific, Germany), a peek spray chamber (Thermo Fischer Scientific, Germany) and a robust Mira Mist peek nebulizer (Burgener, England). The respective elements were analyzed for the following masses: 197Au and 46/47/49Ti. Additionally, the concentration of freeze-dried adult caddisflies (including any nanoparticles potentially adsorbed to the exterior of the organisms) and adult control organisms submersed in either the 400 µg nTiO2/L or the 6.5 µg nAu/L treatment to control for the feasible external adhesion of nanoparticles, as well as composite samples of the leaf material offered as a food source during the experiment, were analyzed via ICP-MS. The nAu samples were analyzed after digestion with aqua regia for 96 h in darkness while the temperature was increased from 30 to 80 °C. All digested samples were diluted in 1% HCl and centrifuged (3000 rpm for 15 min) to remove indigestible residues. Finally, the supernatants were analyzed by ICP-MS as described for water samples. For nTiO2 samples, the device was coupled with electrothermal vaporization (ETV 4000 System, Spectral Systems, Germany) for sample introduction to overcome the poor solubility of TiO244. Prior to vaporization, the samples were incinerated (300 °C) to lower the introduction of organics. Furthermore, the instrument was run in collision cell mode with −2.5 V and 4 mL He/H2 cell gas in order to avoid polyatomic interference. In addition, 25 mL Ar/O2 gas was applied to oxidize the introduced carbon.
TEM analyses
Each nanoparticle stock suspension (nTiO2 and nAu) was diluted and transferred onto a copper grid coated with carbon by ultrasonic nebulization and investigated with the 200 keV Zeiss 922 Omega transmission electron microscope. The caddisflies, in contrast, were fixed in a mixture of 2.5% glutaraldehyde, 1.25% formaldehyde, 0.03% picric acid and 0.003% CaCl2 in 0.1 M cacodylatbuffer (pH 7.4) for 24 h at 4 °C. Subsequently the samples were washed three times with 0.1 M cacodylatbuffer and secondary fixed using 1% OsO4 in 0.1 M cacodylatbuffer for 24 h at 4 °C. Samples were dehydrated by undenatured ethyl alcohol and embedded in epoxy resin (intermediate medium: 1,2-epoxypropane). The abdomen of each fly was cut crosswise with a microtome and each slice was put on a single slot copper grid (oval hole), while leaving out any type of contrasting. The specimens were investigated with the transmission electron microscope and the Quanta 325 environmental scanning electron microscope in wet scanning transmission electron microscopy mode (dark and bright field) taking advantage of the energy dispersive X-ray spectroscopy.
Data evaluation
Emergence over time was expressed as a percent relative to the total number of emerging insects at the end of the experiment (i.e., after 140 days). On the basis of the cumulative emergence data, non-linear regression analysis was performed to identify the time by which 50% of the Chaetopteryx larvae had emerged [with 95% confidence interval (CI)]. For this purpose, several 2-parameter models (generally Weibull or log-normal models) were fitted to the data using the “R” extension package “drc”45. The model fitting the data best was selected on the basis of Akaike’s Information Criterion (i.e., lowest score; drc-function “mselect”) and visual inspection. The time until median emergence was derived using the drc-function “ED”. Obtained values were assessed for statistically significant differences between treatments using CI-testing (drc-function “comped”)46. The concentrations of TiO2, Au and lipids in emerged Chaetopteryx were compared based on their median or mean and (non-)parametric CI-testing methods, as detailed in Altman et al.47. CI-testing is basically a formalized approached to assess for the overlap of variability between treatments48. R version 3.0.3 for Mac was used for statistics and figures49.
Data availability
Data are available from the corresponding authors upon request.
References
Gottschalk, F., Sun, T. Y. & Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 181, 287–300 (2013).
Klaine, S. J. et al. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 27, 1825–1851 (2008).
Mueller, N. C. & Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 42, 4447–4453 (2008).
Ferry, J. L. et al. Transfer of gold nanoparticles from the water column to the estuarine food web. Nature Nanotechnology 4, 441–444 (2009).
Werlin, R. et al. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nature Nanotechnology 6, 65–71 (2011).
Judy, J. D., Unrine, J. M. & Bertsch, P. M. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ. Sci. Technol. 45, 776–781 (2011).
Epanchin, P. N., Knapp, R. A. & Lawler, S. P. Nonnative trout impact an alpine-nesting bird by altering aquatic-insect subsidies. Ecology 91, 2406–2415 (2010).
Uesugi, A. & Murakami, M. Do seasonally fluctuating aquatic subsidies influence the distribution pattern of birds between riparian and upland forests? Ecol. Res. 22, 274–281 (2007).
Pace, M. L. et al. Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature 427, 240–243 (2004).
Al-Jaibachi, R., Cuthbert, R. N. & Callaghan, A. Up and away: ontogenic transference as a pathway for aerial dispersal of microplastics. Biology Letters 14, 20180479 (2018).
Kraus, J. M. et al. Cross-ecosystem impacts of stream pollution reduce resource and contaminant flux to riparian food webs. Ecol. Appl. 24, 235–243 (2014).
Kraus, J. M. et al. Metamorphosis alters contaminants and chemical tracers in insects: implications for food webs. Environ. Sci. Technol. 48, 10957–10965 (2014).
Paetzold, A., Smith, M., Warren, P. H. & Maltby, L. Environmental impact propagated by cross-system subsidy: chronic stream pollution controls riparian spider populations. Ecology 92, 1711–1716 (2011).
Cristol, D. A. et al. The movement of aquatic mercury through terrestrial food webs. Science 320, 335–335 (2008).
Kraus, J. M., Gibson, P. P., Walters, D. M. & Mills, M. A. Riparian spiders as sentinels of polychlorinated biphenyl contamination across heterogeneous aquatic ecosystems. Environ. Toxicol. Chem. 36, 1278–1286 (2017).
Richmond, E. K. et al. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat Commun 9, 4491 (2018).
Schulz, R. et al. Review on environmental alterations propagating from aquatic to terrestrial ecosystems. Sci. Total Environ. 538, 246–261 (2015).
Ulmer, G. In Die Tierwelt Mitteleuropas VI.: XV 1 - XV (eds Brohmer, P., Ehrmann, P. & Ulmer, G.) (1929).
Häder, D. P. et al. ELDONET - a decade of monitoring solar radiation on five continents. Photochem. Photobiol. 83, 1348–1357 (2007).
Fujishima, A., Rao, T. N. & Tryk, D. A. Titanium dioxide photocatalysis. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 1, 1–21 (2000).
Feckler, A., Rosenfeldt, R. R., Seitz, F., Schulz, R. & Bundschuh, M. Photocatalytic properties of titanium dioxide nanoparticles affect habitat selection of and food quality for a key species in the leaf litter decomposition process. Environ. Pollut. 196, 276–283 (2015).
Gondikas, A. P. et al. Release of TiO2 nanoparticles from sunscreens into surface waters: a one-year survey at the old Danube recreational lake. Environ. Sci. Technol. 48, 5415–5422 (2014).
Kiser, M. A. et al. Titanium nanomaterial removal and release from wastewater treatment plants. Environ. Sci. Technol. 43, 6757–6763 (2009).
Nam, S. H. et al. Derivation of guideline values for gold (III) ion toxicity limits to protect aquatic ecosystems. Water Res. 48, 126–136 (2014).
Tiede, K., Hassellov, M., Breitbarth, E., Chaudhry, Q. & Boxall, A. B. A. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 1216, 503–509 (2009).
Baxter, C. V., Fausch, K. D. & Saunders, C. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zone. Freshw. Biol. 50, 201–220 (2005).
Jackson, J. K. & Fisher, S. G. Secondary production, emergence, and export of aquatic insects of a sonoran desert stream. Ecology 67, 629–638 (1986).
Strasevicius, D., Jonsson, M., Nyholm, N. E. I. & Malmqvist, B. Reduced breeding success of Pied Flycatchers Ficedula hypoleuca along regulated rivers. Ibis 155, 348–356 (2013).
Mahler, G. J. et al. Oral exposure to polystyrene nanoparticles affects iron absorption. Nature Nanotechnology 7, 264–271 (2012).
Kurta, A., Bell, G. P., Nagy, K. A. & Kunz, T. H. Energetics of pregnancy and lactation in free- ranging little brown bats Myotis lucifugus. Physiol. Zool. 62, 804–818 (1989).
Aguilar, F. et al. Scientific Opinion on the re-evaluation of gold (E 175) as a food additive. Efsa Journal 14 (2016).
Hadrup, N., Sharma, A. K., Poulsen, M. & Nielsen, E. Toxicological risk assessment of elemental gold following oral exposure to sheets and nanoparticles - A review. Regul. Toxicol. Pharmacol. 72, 216–221 (2015).
Bundschuh, M., Seitz, F., Rosenfeldt, R. R. & Schulz, R. Effects of nanoparticles in fresh waters – risks, mechanisms and interactions. Freshw. Biol. 61, 2185–2196 (2016).
Raitif, J., Plantegenest, M., Agator, O., Piscart, C. & Roussel, J.-M. Seasonal and spatial variations of stream insect emergence in an intensive agricultural landscape. Sci. Total Environ. 644, 594–601 (2018).
Piccinno, F., Gottschalk, F., Seeger, S. & Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. Journal of Nanoparticle Research 14, 1109 (2012).
Keller, A. A. & Lazareva, A. Predicted releases of engineered nanomaterials: from global to regional to local. Environmental Science & Technology Letters 1, 65–70 (2014).
Dabrunz, A. et al. Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. PLoS One 6, e20112 (2011).
McFarland, A. D., Haynes, C. L., Mirkin, C. A., Van Duyne, R. P. & Godwin, H. A. Color my nanoworld. Journal of Chemical Education 81, 544–545 (2004).
Borgmann, U. Systematic analysis of aqueous ion requirements of Hyalella azteca: A standard artificial medium including the essential bromide ion. Arch. Environ. Contam. Toxicol. 30, 356–363 (1996).
Kalčíková, G. et al. Combined effect of UV-irradiation and TiO2-nanoparticles on the predator-prey interaction of gammarids and mayfly nymphs. Environ. Pollut. 186, 136–140 (2014).
Bundschuh, M. & Schulz, R. Population response to ozone application in wastewater: an on-site microcosm study with Gammarus fossarum (Crustacea: Amphipoda). Ecotoxicology 20, 466–473 (2011).
Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1, 302–304 (1985).
Rosenfeldt, R. R., Seitz, F., Schulz, R. & Bundschuh, M. Heavy metal uptake and toxicity in the presence of titanium dioxide nanoparticles: A factorial approach using Daphnia magna. Environ. Sci. Technol. 48, 6965–6972 (2014).
Duester, L., Prasse, C., Vogel, J. V., Vink, J. P. M. & Schaumann, G. E. Translocation of Sb and Ti in an undisturbed floodplain soil after application of Sb2O3 and TiO2 nanoparticles to the surface. Journal of Environmental Monitoring 13, 1204–1211 (2011).
Ritz, C. & Streibig, J. C. Bioassay analysis using R. Journal of Statistical Software 12, 1–22 (2005).
Wheeler, M. W., Park, R. M. & Bailer, A. J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 25, 1441–1444 (2006).
Altman, D. G., Machin, D., Bryant, T. N. & Gardner, M. J. Statistics with confidence - 2nd edition. (British Medical Journal, 2000).
Newman, M. C. “What exactly are you inferring?” A closer look at hypothesis testing. Environ. Toxicol. Chem. 27, 1013–1019 (2008).
R Developmental Core Team, R Foundation for Statistical Computing (2015).
Acknowledgements
We acknowledge T. Bürgi for her assistance in the laboratory, C. Schilde for providing the nTiO2 suspension, G. Jürges and M. Ardakani for their help with TEM and J. Kucerik for his support with ESEM. This study is supported by the research group INTERNANO funded by the German Research Foundation (DFG; SCHU2271/5) and the Ministry for Education, Science, Further Education and Culture Rhineland-Palatinate (MBWWK). We acknowledge the Fix-Stiftung, Landau for additional financial support. Open access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
M.B. and R.S. conceived and designed the experiment and initiated the analysis. M.B. and D.E. directed the experiment. R.R.R. performed the ICP-MS measurements. R.R.R. and F.S. performed the nanoparticle analysis. D.E., R.R.R., R.B., A.F., S.L., F.S. and J.Z. jointly performed the experiment. M.B., D.E., J.Z. and R.S. conducted the statistical analyses. M.B. and R.S. prepared the first version of the manuscript and handled its submission. D.E. and R.R.R. contributed parts of the methods section. All authors discussed the manuscript and contributed to the editing.
Corresponding authors
Ethics declarations
Competing interests
Some of the authors (R.R.R., F.S., R.S.) are managing directors of small consultancies working in the field of ecotoxicology and environmental risk assessment or are now employed as consultant (D.E.). The authors, however, do not feel a conflict of interest as a consequence of this situation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bundschuh, M., Englert, D., Rosenfeldt, R.R. et al. Nanoparticles transported from aquatic to terrestrial ecosystems via emerging aquatic insects compromise subsidy quality. Sci Rep 9, 15676 (2019). https://doi.org/10.1038/s41598-019-52096-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52096-7
This article is cited by
-
Recent Insights into the Silver Nanomaterials: an Overview of Their Transformation in the Food Webs and Toxicity in the Aquatic Ecosystem
Water, Air, & Soil Pollution (2023)
-
Colloidal stability and aggregation kinetics of nanocrystal CdSe/ZnS quantum dots in aqueous systems: effects of pH and organic ligands
Journal of Nanoparticle Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.