Abstract
Deficiency of vitamin D is associated with increased risk of several types of cancer including colorectal cancer (CRC). However, factors contributing to low levels of 25-hydroxyvitamin D [25(OH)D] in CRC are not clear. Therefore, in this study serum 25(OH)D levels in 117 CRC patients and 86 controls were analyzed and correlated with the clinicopathological data including morphological subtype (serrated or conventional), quantity of tumor infiltrating immune cells, levels of systemic inflammatory markers, and disease outcome. We found that the patients had lower serum 25(OH)D levels compared to the controls. Interestingly, among the patients mismatch repair deficiency, serrated morphology, and high body mass index associated with lowest serum 25(OH)D levels. In addition, patients operated in summer or autumn had higher serum 25(OH)D levels. Furthermore, serum 25(OH)D levels inversely correlated with several systemic inflammatory markers, e.g. serum C reactive protein, but did not associate with prognosis. Mechanism leading to vitamin D deficiency in these patients are not clear but could be related to the effects of systemic inflammation. Longitudinal studies are warranted to assess vitamin D deficiency as a potential risk factor for serrated colorectal polyps and adenocarcinoma.
Similar content being viewed by others
Introduction
Acquired from the diet or synthesis in the skin under sunlight exposure, vitamin D is hydroxylated in the liver into the major circulating form, 25-hydroxyvitamin D [25(OH)D], which is commonly used to determine the patients’ vitamin D status1,2. There is no universally accepted definition of the normal range of human 25(OH)D levels, but levels of 30–100 ng/ml (75 to 250 nmol/L) are considered to fall within the normal limits and levels of 0 to 20 ng/ml (0–50 nmol/L) are considered deficient. The hydroxylation of 25(OH)D into the hormonally active form of vitamin D (1,25(OH)2D3) takes place in the kidneys and also in most extrarenal tissues, where it acts in a paracrine manner1. The hormonally active form, 1,25(OH)2D3, has a short half-life and tight homeostatic control1. The classical role of vitamin D is to regulate mineral homeostasis and to control bone metabolism, while other functions include the regulation of immune responses, the induction of cell differentiation, the stimulation of apoptosis, and the inhibition of cell proliferation, angiogenesis, and metastasis3,4,5.
Colorectal cancer (CRC) is the second most common fatal malignancy in the Western world. CRC is a multi-pathway disease, and 10–30% of the cases are considered to develop from the serrated colorectal polyps and evolve along the serrated pathway6. Serrated colorectal adenocarcinoma (SAC) can be distinguished by its characteristic morphology6, which reflects its unique messenger RNA expression profile compared to conventional colorectal adenocarcinoma (CC)7.
Vitamin D deficiency has been associated with a variety of cancers1,3, and epidemiological studies have also demonstrated an association between vitamin D deficiency and an increased risk of colorectal cancer (CRC)8,9. Moreover, low plasma prediagnostic10 and postoperative11 25(OH)D levels in CRC patients have been associated with adverse prognosis, according to meta-analyses12,13. However, the determinants of preoperative 25(OH)D levels are incompletely known14. Although vitamin D has been linked with an anti-inflammatory function3, the associations between preoperative serum 25(OH)D levels in CRC and tumor associated immune/inflammatory cell reaction or systemic levels of the inflammatory mediators and markers have not been well-characterized. Finally, there is no information about the association between serum 25(OH)D levels and different pathways of CRC development.
In this study, we have analyzed the preoperative serum 25(OH)D levels in a series of 117 prospectively recruited CRC patients and 86 healthy matched controls in Northern Finland (latitude 65° North). Especially, the aim was to characterize the association of serum 25(OH)D levels with the developmental route, with details of local and systemic inflammatory reaction patterns, and with survival.
Results
Serum 25(OH)D in CRC patients and healthy controls
There were no significant differences in the average age or sex distribution between the CRC patients and the controls (Table S1). The median body mass index (BMI) of the patients was 26.3, while no data on BMI was available for controls aged less than 65 (healthy blood donor group). However, there was no significant difference in the BMI of the patients aged 65 or more compared to the respective controls (median 26.6 vs. 26.9, p = 0.205). The patients had significantly lower serum 25(OH)D levels relative to the controls (median 49.0 nmol/L vs. 59.5 nmol/L, p = 6.6E-5). Receiver operating characteristics (ROC) analysis indicated an area under the curve (AUC) of 0.662 (95% CI 0.59-0.74) for serum 25(OH)D in the discrimination of the cases and controls, and using a cut-off of 50 nmol/L, the sensitivity was 80.2% and the specificity was 52.1%
Serum 25(OH)D levels and clinical and pathological characteristics
Serum 25(OH)D levels did not significantly correlate with patient age (p = 0.746) or gender (p = 0.204), tumor location (p = 0.116), TNM stage (p = 0.420), and WHO grade (p = 0.205) (Table 1). However, the patients with body BMI > 30 had lower serum 25(OH)D levels relative to those with BMI ≤ 30 (p = 0.0032), and also the patients operated in winter or spring had lower serum 25(OH)D levels (p = 0.012). SAC associated with decreased serum 25(OH)D levels relative to the CC (p = 0.029). Mismatch repair (MMR) deficiency is characteristic to Lynch syndrome (hereditary nonpolyposis colorectal cancer) and frequent in the serrated route of CRC6, and was associated with reduced serum 25(OH)D levels (p = 0.018). However, the presence of BRAF or KRAS mutation, also most frequently observed in SAC6, did not significantly correlate with serum 25(OH)D levels (p = 0.512).
Serum 25(OH)D, immune cell infiltration, and systemic inflammatory markers
To evaluate the potential effects of the immune-modulating functions of Vitamin D in CRC, we analyzed the associations between serum 25(OH)D levels and systemic inflammatory markers (Table 2), as well as local inflammatory cell densities in CRC tissue (Table S2). Serum 25(OH)D levels inversely correlated with an assemblage of systemic inflammatory markers, most notably with blood neutrophil count (p = 0.0012), serum C-reactive protein (CRP) levels (p = 0.0021), blood neutrophil/lymphocyte ratio (NLR) (p = 0.0041), and serum interleukin (IL)-6 levels (p = 0.0042) (Table 2). Instead, of the studied types of tumor infiltrating immune cells, only intratumoral CD1a+ dendritic cells (p = 0.012) and neutrophils (p = 0.027) showed significant correlation with serum 25(OH)D levels (Table S2).
Multiple linear regression models
Multiple linear regression modeling was utilized to evaluate the independent significance of different explanatory variables on serum 25(OH)D levels (Table 3). The models indicated that sunlight exposure, i.e. serum samples taken during winter or spring, serrated histology, and blood neutrophil count were independent predictors of low serum 25(OH)D levels.
Serum 25(OH)D and survival
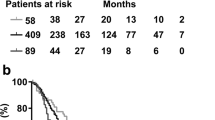
Finally, we evaluated the association between serum 25(OH)D levels and disease outcome. ROC analysis indicated an AUC of 0.567 (95% CI 0.419–0.716) for the detection of patients with recurrent disease in 60 month follow-up. Further analyses utilizing different cut-of points ranging from 30 to 70 nmol/L indicated that serum 25(OH)D did not significantly associate with disease-free, cancer-specific, or overall survival in 60 month follow-up (Table S3). Kaplan-Meier curves with a cut-off point of 50 nmol/L are presented as Fig. 1.
Kaplan-Meier curves demonstrating the associations between serum 25(OH)D levels and disease-free survival (DFS), cancer-specific survival (CSS), and overall survival (OS).
(A) Serum 25(OH)D and DFS. (B) Serum 25(OH)D and CSS. (C) Serum 25(OH)D and OS. Abbreviations: CI: confidence interval; HR: hazard ratio.
Discussion
In recent decades, the deficiency of vitamin D has been implicated in several chronic metabolic, cardiovascular, and neoplastic diseases3. The present study investigated the factors contributing to preoperative serum 25(OH)D levels in CRC and their significance. According to our observations, the development of cancer by the serrated pathway, systemic inflammation, and obesity are associated with vitamin D deficiency in CRC.
Our study indicates that CRC patients have lower serum 25(OH)D levels as compared to healthy controls. Earlier studies have associated low serum 25(OH)D levels with an increased risk of developing CRC8,9, while the results of the studies assessing preoperative values in cancer patients have been contradictory15,16. Interestingly, the median serum 25(OH)D levels in stage I patients did not differ from the levels of healthy controls, but low median serum 25(OH)D levels were associated to the stage II-IV CRC. This finding suggests that the decrease in serum 25(OH)D may be related to the progression of CRC, also supported by some earlier results15.
The progression of cancer is associated with the activation of systemic inflammatory response that may regulate and promote metastasis17. In CRC, systemic inflammatory response, as evidenced by increased serum CRP and decreased serum albumin (mGPS) or increased blood neutrophil/lymphocyte ratio, indicates adverse prognosis17. We report here of an association between low serum 25(OH)D levels and high scores of systemic inflammatory response in CRC. Similar association has been found in healthy adults and general patient material18. This association may reflect the immunomodulatory and immunosuppressive functions of vitamin D19, proinflammatory cytokines suppressing hepatic production of carrier proteins of vitamin D, or redistribution or consumption of vitamin D storages by the systemic inflammatory response18. The results support the suggestion by Conway and McMillan20 that some of the studies addressing the association between circulating 25(OH)D concentrations and CRC outcome may have been confounded by the effect of systemic inflammatory response. Indeed, also in the multivariate analysis, the systemic inflammatory response, as indicated by high blood neutrophil count, had a higher association with low serum 25(OH)D levels than high BMI.
Immune cell infiltration has frequently been associated with improved survival in CRC21,22. It was recently proposed that prediagnostic vitamin D deficiency is a risk factor for CRC with intense intratumoral periglandular immune reaction23. Our analyses indicate that serum 25(OH)D concentrations positively correlate with the densities of CD1a+ dendritic cells and neutrophils at the tumor stroma but not with T cells that are considered more important in tumor immunosurveillance and have better-established prognostic value21,22. The mechanism and the significance of the association we observed is not clear, but may be related to the well accepted immunoregulatory role of 25(OH)D on dendritic cells24 and requires further investigation. However, our findings do not suggest that tumor inflammatory infiltrate, in overall, is an important determinant of serum 25(OH)D levels in CRC patients or vice versa.
The patients with tumors showing serrated histology had lower serum 25(OH)D levels as compared with those with non-serrated histology. Similar association was seen with MMR deficiency, a characteristic feature of SAC. These associations suggest that vitamin D deficiency might be related to the serrated pathway of CRC. Earlier studies have reported that vitamin D deficiency is not a risk factor for colorectal hyperplastic polyps25, but to our knowledge, little is known of the association between vitamin D deficiency and sessile and traditional serrated adenomas, which is an important subject for further investigation, since hyperplastic polyps are very common, and the risk of neoplastic progression has not been attributed to hyperplastic polyps but sessile and traditional serrated adenomas6.
Our data indicates an inverse correlation between serum 25(OH)D levels and BMI in CRC patients, and earlier studies support similar association in healthy adults26,27. The subcutaneous fat may store more vitamin D synthesized in the skin, and obese persons have significantly lower increase in serum 25(OH)D after UV-B exposure26. Moreover, obesity is associated with low level of physical activity, which may increase the time spend inside, thus decreasing UV exposure and serum 25(OH)D levels. As vitamin D deficiency, obesity is also associated with increased risk of CRC, and it has been suggested that vitamin D deficiency in obese people may explain at least 20% of cancer risk attributable to high BMI28.
There is variation in circulating 25(OH)D in Finnish population due to the seasonal changes in the exposure to sunlight and low vitamin D intake29,30. Hassi et al.31 have evaluated the luminosity in our latitude and only from June to October, the luminosity is sufficient for Vitamin D production in the skin. Accordingly, our results indicate that patients operated in winter or spring have significantly higher likelihood of low serum 25(OH)D, and the season of operation was the most important predictor of 25(OH)D in the multivariate analyses. This result points out the importance to take this confounding factor into account also in the subsequent studies. Whether this seasonal variation in serum levels of 25(OH)D has clinical significance in cancer patients, is not well-known. This study did not include any dietary surveys but previous studies have reported that the intake of vitamin D in the area is most frequently below the recommended intake32. A previously published dietary survey of 8960 subjects born in 1966 in the Northern Finland indicated that only roughly one fourth had regular fish intake33. Moreover, in a Nordic multi-center trial with subjects (n = 213) with metabolic syndrome randomized to a control or a healthy Nordic diet favoring fish (≥300 g/week, including ≥200 g/week fatty fish) among other healthy Nordic products34, the healthy Nordic diet intervention increased vitamin D intake but not plasma 25(OH)D concentration. The reason, why fish consumption did not improve vitamin D status might be that many fish are farmed and might contain little vitamin D or that frying fish may result in vitamin D destruction35. All in all, the lack of dietary surveys is a limitation of this study, which could cause confounding of the results, since for example, the use of vitamin D supplements could increase serum 25(OH)D levels.
There are also other limitations have to be taken account, when interpreting the results of the study. First, no surveys on physical activity were conducted, and we cannot rule out the effect of physical activity. However, we did not observe any difference between serum 25(OH)D levels in stage II-IV patients (Table 1). All patients participating this study were eligible to surgery. Therefore it is unlikely that physical inactivity due to disease burden would play a major role in explaining decreased serum 25(OH)D levels. Second, the BMI is another potential confounding factor, and no BMI data was available for controls aged less than 65. Nevertheless, the BMI of the patients aged 65 or more did not differ from that of the respective controls, and also within the CRC patient subgroup, the multivariate analysis indicated that the associations between lower serum 25(OH)D levels and systemic inflammation and serrated histology were independent of the BMI of the patients. The advantages of the study included a prospectively recruited, well-characterized study population including patients from different stages and with a plethora of analyzed markers of systemic and local inflammatory response. The patients were from a single surgical unit and had uniform follow-up schedule.
In conclusion, decreased serum 25(OH)D levels in CRC are associated with serrated tumor morphology, and systemic inflammatory response. Further experimental studies are warranted to address biological mechanisms underlying the findings and possible role of low vitamin D levels in the development of serrated CRC.
Patients and Methods
Patients and controls
All newly diagnosed CRC patients operated in Oulu University Hospital, between April 2006 and January 2010 (n = 344) were introduced for this prospective study. The study design was approved by the Ethical Committee of Oulu University Hospital (58/2005, 184/2009), and the methods were performed in accordance with the relevant guidelines and regulations. Preoperative blood samples and surgical specimens were originally collected from 149 patients, who had signed an informed consent to participate and were eligible to the study36. 32 of 149 patients (21.5%) received preoperative radiotherapy or chemoradiotherapy (RT/CRT) and were excluded from the analyses since RT/CRT is a potential confounding factor that may affect the histological characteristics of the tumors37 and associates with vitamin D deficiency38. Patients were followed up in regular intervals for up to five years39,40. The REporting recommendations for tumor MARKer prognostic studies (REMARK) were taken into account in the study design and reporting41.
Age and sex matched control serum samples were acquired from healthy voluntary blood donors (Finnish Red Cross, Oulu, Finland; n = 36, age < 65 years) and cataract surgery patients (Oulu University Hospital; n = 50, age ≥ 65 years). Blood samples from patients and controls were collected and centrifuged, and the serum was stored at −70 °C until further analysis. The study set-up is previously described36. The data of the BMI of the patients and the controls aged ≥ 65 years was collected from the clinical records, while no such data was available for controls aged < 65 years36.
Determination of serum 25(OH)D levels and systemic inflammation
Serum 25(OH)D levels were measured using 25-Hydroxy vitamin D enzyme immunoassay (EIA) kit (Immunodiagnostic Systems GmbH, Germany) following manufacturer’s instructions and described previously42. For accuracy assessment, each measurement was included with certified materials from National Institute of Standards and Technology, United States (NIST, USA); Standard Reference Material-972 (SRM-972; consists four vials from level 1 to 4) and two internal plasma controls43. The inter-assay variations (CVs) ranged within (8.6 to 14.6%) for different control and certified materials. All the measurements were performed blinded to the clinical data.
Differential leukocyte count, serum CRP, and serum albumin was analyzed in the laboratory of Oulu University hospital36,44, and mGPS was calculated from CRP and albumin values36. Concentrations of serum levels of thirteen cytokines were measured as described earlier36.
Histopathological analyses of the tumors and associated inflammatory and immune cell reaction and determination of KRAS and BRAF mutations
Tumors were classified according to TNM6 classification45 and their differentiation evaluated according to the WHO criteria46. Colorectal cancer associated lymphoid reaction (CLR), denoting lymphoid follicles surrounding the tumors, was assessed as “the number of lymphoid follicles/the length of the invasive front of the tumor” as described earlier47. Tumors were classified into SACs and CCs by the established criteria, as described earlier46,48.
MMR enzyme status was evaluated utilizing MLH1 and MSH2 immunohistochemistry44. Utilizing a tissue microarray with one to four (median three) cores of 3.0 mm diameter per case from the invasive margin (IM) and the center of the tumor (CT)49, the densities of the immune cell infiltrate at the IM, and the CT (stroma, CT-S; intraepithelial, CT-IEL) were analyzed using ImageJ, a free image analysis software, and a computer assisted counting method as described earlier49,50. BRAF and KRAS mutation analysis was carried out as described earlier48.
Statistical analyses
Normally distributed continuous variables are presented as mean (standard deviation, SD), whereas other continuous variables are presented as median (interquartile range, IQR). IBM SPSS Statistics for Windows version 22.0 (IBM Corp. Armonk, NY) was used for the statistical analyses. Statistical significances of the associations between serum 25(OH)D levels and categorical variables were analyzed by Mann-Whitney U test (comparing two classes) or Kruskal-Wallis test (comparing three or more classes). Pearson correlation coefficients (r) were used to assess the correlations between two continuous variables. Logarithmic transformation was applied to variables with positive skewness. Multiple linear regression analysis was used to model the relationship between serum 25(OH)D levels and several explanatory variables. Kaplan-Meier method and Log rank test were utilized in the survival analyses. A two-tailed p < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Väyrynen, J. P. et al. Decreased preoperative serum 25-Hydroxyvitamin D levels in colorectal cancer are associated with systemic inflammation and serrated morphology. Sci. Rep. 6, 36519; doi: 10.1038/srep36519 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007).
Huotari, A. & Herzig, K.-H. Vitamin D and living in northern latitudes--an endemic risk area for vitamin D deficiency. Int. J. Circumpolar Health 67, 164–178 (2008).
Rosen, C. J. et al. The nonskeletal effects of vitamin D: An endocrine society scientific statement. Endocr. Rev. 33, 456–492 (2012).
Mutt, S. J., Hyppönen, E., Saarnio, J., Järvelin, M.-R. & Herzig, K.-H. Vitamin D and adipose tissue-more than storage. Front. Physiol. 5, 228 (2014).
Meeker, S. et al. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 74, 4398–4408 (2014).
Mäkinen, M. J. Colorectal serrated adenocarcinoma. Histopathology 50, 131–150 (2007).
Laiho, P. et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene 26, 312–320 (2007).
Gorham, E. D. et al. Optimal Vitamin D Status for Colorectal Cancer Prevention. A Quantitative Meta Analysis. Am. J. Prev. Med. 32, 210–216 (2007).
Lee, J. E. et al. Circulating levels of vitamin D and colon and rectal cancer: The Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev. Res. 4, 735–743 (2011).
Ng, K. et al. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J. Clin. Oncol. 26, 2984–2991 (2008).
Zgaga, L. et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J. Clin. Oncol. 32, 2430–2439 (2014).
Maalmi, H., Ordóñez-Mena, J. M., Schöttker, B. & Brenner, H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur. J. Cancer 50, 1510–1521 (2014).
Li, M. et al. Review: The Impacts of Circulating 25-Hydroxyvitamin D Levels on Cancer Patient Outcomes: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 99, jc20134320 (2014).
Mezawa, H. et al. Serum vitamin D levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC Cancer 10, 347 (2010).
Niv, Y. et al. In colorectal carcinoma patients, serum vitamin D levels vary according to stage of the carcinoma. Cancer 86, 391–397 (1999).
Charalampopoulos, A. et al. Parathormone and 1,25(OH)2D3 but not 25(OH)D3 serum levels, in an inverse correlation, reveal an association with advanced stages of colorectal cancer. Clin. Exp. Med. 10, 69–72 (2010).
McMillan, D. C. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat. Rev. 39, 534–540 (2013).
Ghashut, R. A., Talwar, D., Kinsella, J., Duncan, A. & McMillan, D. C. The effect of the systemic inflammatory response on plasma vitamin 25 (OH) D concentrations adjusted for albumin. PLoS One 9, 1–7 (2014).
Mutt, S. J. et al. Inhibition of cytokine secretion from adipocytes by 1,25-dihydroxyvitamin D3 via the NF-κB pathway. FASEB J. 26, 4400–4407 (2012).
Conway, F. J. S. & McMillan, D. C. Plasma Vitamin D Concentration and Survival in Colorectal Cancer: Potential Confounding by the Systemic Inflammatory Response. J. Clin. Oncol. 33, 224–224 (2015).
Roxburgh, C. S. & McMillan, D. C. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat. Rev. 38, 451–466 (2012).
Väyrynen, J. P. et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int. J. Cancer 139, 112–121 (2016).
Song, M. et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut, doi: 10.1136/gutjnl-2014-308852 (2015).
Bscheider, M. & Butcher, E. C. Vitamin D immunoregulation through Dendritic Cells. Immunology, doi: 10.1111/imm.12610 (2016).
Adams, S. V. et al. Circulating 25-hydroxyvitamin-D and risk of colorectal adenomas and hyperplastic polyps. Nutr. Cancer 63, 319–326 (2011).
Wortsman, J., Matsuoka, L. Y., Chen, T. C., Lu, Z. & Holick, M. F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 72, 690–693 (2000).
Vimaleswaran, K. S. et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 10, e1001383 (2013).
Lagunova, Z., Porojnicu, A. C., Grant, W. B., Bruland, Ø. & Moan, J. E. Obesity and increased risk of cancer: Does decrease of serum 25-hydroxyvitamin D level with increasing body mass index explain some of the association? Mol. Nutr. Food Res. 54, 1127–1133 (2010).
Bolland, M. J. et al. The effects of seasonal variation of 25-hydroxyvitamin D on diagnosis of vitamin D insufficiency. N. Z. Med. J. 121, 63–74 (2008).
Savolainen, K., Maenpaa, P. H., Alhava, E. M. & Kettunen, K. A seasonal difference in serum 25-hydroxyvitamin D3 in a Finnish population. Med. Biol. 58, 49–52 (1980).
Hassi, J., Sikkila, K., Ruokonen, A. & Leppaluoto, J. The pituitary-thyroid axis in healthy men living under subarctic climatological conditions. J. Endocrinol. 169, 195–203 (2001).
Jonsdottir, S. E. et al. Adherence to the Nordic Nutrition Recommendations in a Nordic population with metabolic syndrome: high salt consumption and low dietary fibre intake (The SYSDIET study). Food Nutr Res 57, 1–11 (2013).
Kelloniemi, H., Ek, E. & Laitinen, J. Optimism, dietary habits, body mass index and smoking among young Finnish adults. Appetite 45, 169–176 (2005).
Uusitupa, M. et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome – a randomized study (SYSDIET). J. Intern. Med. 274, 52–66 (2013).
Brader, L. et al. Effects of a healthy Nordic diet on plasma 25-hydroxyvitamin D concentration in subjects with metabolic syndrome: a randomized, [corrected] controlled trial (SYSDIET). Eur. J. Nutr. 53, 1123–1134 (2014).
Kantola, T. et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br. J. Cancer 107, 1729–1736 (2012).
Nagtegaal, I. D. et al. Short-term preoperative radiotherapy interferes with the determination of pathological parameters in rectal cancer. J. Pathol. 197, 20–27 (2002).
Fakih, M. G. et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int. J. Colorectal Dis. 24, 219–224 (2009).
Kantola, T. et al. Serum endostatin levels are elevated in colorectal cancer and correlate with invasion and systemic inflammatory markers. Br. J. Cancer 111, 1605–1613 (2014).
Moilanen, J. M. et al. Collagen XVII expression correlates with the invasion and metastasis of colorectal cancer. Hum. Pathol. 46, 434–442 (2015).
McShane, L. M. et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur. J. Cancer 41, 1690–1696 (2005).
Ala-Kokko, T. I. et al. Vitamin D deficiency at admission is not associated with 90-day mortality in patients with severe sepsis or septic shock: Observational FINNAKI cohort study. Ann. Med. 48, 67–75 (2016).
Wallace, A. M., Gibson, S., de la Hunty, A., Lamberg-Allardt, C. & Ashwell, M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids 75, 477–488 (2010).
Väyrynen, J. P. et al. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int. J. Cancer 131, E463–E474 (2012).
Sobin, L. H. & Wittekind, C. TNM classification of malignant tumours. (Wiley-Liss, 2002).
Hamilton, S. R. et al. In WHO classification of tumours of the digestive system. (eds Bosman, F. T., Carneiro, F., Hruban, R. H. & Theise, N. D. ) 134–146 (IARC Press, 2010).
Väyrynen, J. P. et al. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int. J. Cancer 134, 2126–2135 (2014).
Sajanti, S. A. et al. VE1 immunohistochemistry accurately detects BRAF V600E mutations in colorectal carcinoma and can be utilized in the detection of poorly differentiated colorectal serrated adenocarcinoma. Virchows Arch. 464, 637–643 (2014).
Väyrynen, J. P. et al. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br. J. Cancer 109, 1839–1847 (2013).
Väyrynen, J. P. et al. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch. 460, 455–465 (2012).
Acknowledgements
We wish to thank Ms. Riitta Vuento for her invaluable assistance in the preparation of the study material. This work was supported by grants from Academy of Finland, Emil Aaltonen Foundation, and Finnish Cancer Foundation.
Author information
Authors and Affiliations
Contributions
Study conception and design: K.-H. Herzig, M. J. Mäkinen, A. Tuomisto. Data collection: J. P. Väyrynen, S. J. Mutt, K.-H. Herzig, S. A. Väyrynen, T. Kantola, T. Karhu, T. J. Karttunen, K. Klintrup, J. Mäkelä, M. J. Mäkinen, A. Tuomisto. Statistical analysis: J. P. Väyrynen, A. Tuomisto. Manuscript draft: J. P. Väyrynen, A. Tuomisto. Manuscript review and editing: J. P. Väyrynen, S. J. Mutt, K.-H. Herzig, S. A. Väyrynen, T. Kantola, T. Karhu, T. J. Karttunen, K. Klintrup, J. Mäkelä, M. J. Mäkinen, A. Tuomisto.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Väyrynen, J., Mutt, S., Herzig, KH. et al. Decreased preoperative serum 25-Hydroxyvitamin D levels in colorectal cancer are associated with systemic inflammation and serrated morphology. Sci Rep 6, 36519 (2016). https://doi.org/10.1038/srep36519
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36519
This article is cited by
-
The association between preoperative 25-OH vitamin D levels and postoperative complications in patients undergoing colorectal cancer surgery
BMC Surgery (2021)
-
Platelet count, aspirin use, and characteristics of host inflammatory responses in colorectal cancer
Journal of Translational Medicine (2019)
-
Serum enterolactone concentrations are low in colon but not in rectal cancer patients
Scientific Reports (2019)
-
Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.