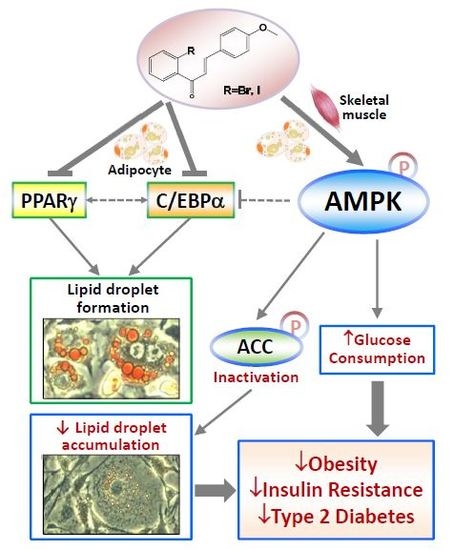
2-Bromo-4′-methoxychalcone and 2-Iodo-4′-methoxychalcone Prevent Progression of Hyperglycemia and Obesity via 5′-Adenosine-Monophosphate-Activated Protein Kinase in Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Results
2.1. Halogen Chalcone Derivatives Promote Glucose Consumption and Reduce Lipid Accumulation in Adipocytes
2.2. Glucose-Lowering Activities of Halogen Chalcone Derivatives Are Associated with AMPK
2.3. Compounds 5 and 6 Decrease Cellular Lipids via Inhibition of PPARγ and C/EBPα
2.4. Compounds 5 and 6 Promote Glucose Consumption in C2C12 Myotubes
2.5. Compounds 5 and 6 Prevent Progression of Hyperglycemia and Obesity in HF-Fed Mice
2.6. Compounds 5 and 6 Prevent Progression of Hyperglycemia and Obesity via AMPK Activation in HF-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of Compounds
4.3. Cell Culture and Differentiation
4.4. Detection of Glucose Consumption
4.5. Oil-Red O Staining
4.6. Animal Studies
4.7. Oral Glucose Tolerance Test
4.8. SDS-PAGE and Western Blotting
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | 5′-adenosine-monophosphate-activated protein kinase |
| ACC | acetyl-CoA carboxylase 1 ACC |
| Akt | Protein kinase B |
| PPARγ | Peroxisome proliferator activated receptor γ |
| C/EBP | CCAAT/enhancer binding protein |
References
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world-a growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.E. Metabolic disease drug discovery- “hitting the target” is easier said than done. Cell Metab. 2012, 15, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Morita, H. Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat. Prod. Rep. 2010, 27, 809–838. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Hsieh, T.J.; El-Shazly, M.; Chuang, D.W.; Tsai, Y.H.; Yen, C.T.; Wu, S.F.; Wu, Y.C.; Chang, F.R. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg. Med. Chem. Lett. 2012, 22, 3912–3915. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Srivastava, S.P.; Srivastava, R.; Rawat, A.K.; Srivastava, A.K.; Pratap, R. Synthesis and antidyslipidemic activity of chalcone fibrates. Bioorg. Med. Chem. Lett. 2011, 21, 3475–3478. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Zheng, C.J.; Sun, L.P.; Liu, X.K.; Piao, H.R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur. J. Med. Chem. 2011, 46, 3469–3473. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Chang, C.C.; Du, Y.C.; Chang, F.R.; Wu, Y.C.; Chang, W.C.; Hsieh, T.J. 2-hydroxy-4′-methoxychalcone inhibits proliferation and inflammation of human aortic smooth muscle cells by increasing the expression of peroxisome proliferator-activated receptor gamma. J. Cardiovasc. Pharmacol. 2012, 59, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Kao, T.Y.; Ko, J.L.; Tzeng, Y.M. Chalcone HTMC causes in vitro selective cytotoxicity, cell-cycle G1 phase arrest through p53-dependent pathway in human lung adenocarcinoma A549 cells, and in vivo tumor growth suppression. Bioorg. Med. Chem. Lett. 2010, 20, 6508–6512. [Google Scholar] [CrossRef] [PubMed]
- Akcok, I.; Cagir, A. Synthesis of stilbene-fused 2′-hydroxychalcones and flavanones. Bioorg. Chem. 2010, 38, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.H.; Park, S.Y.; Kim-Pak, Y.; Lee, H.K.; Park, K.S.; Shin, K.H.; Keum, S.R.; Lim, S.S. Synthesis and PPAR-gamma ligand-binding activity of the new series of 2′-hydroxychalcone and thiazolidinedione derivatives. Chem. Pharm. Bull. (Tokyo) 2006, 54, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Veledo, S.; Vazquez-Carballo, A.; Vila-Bedmar, R.; Ceperuelo-Mallafre, V.; Vendrell, J. Role of energy- and nutrient-sensing kinases AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in adipocyte differentiation. IUBMB Life 2013, 65, 572–583. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.M. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, M.; Mortensen, B.; Pehmoller, C.; Birk, J.B.; Wojtaszewski, J.F. Exercise-induced AMPK activity in skeletal muscle: Role in glucose uptake and insulin sensitivity. Mol. Cell. Endocrinol. 2013, 366, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Zeng, X.Y.; He, Y.; Liu, H.; Wang, B.; Zhou, H.; Chen, J.W.; Liu, P.Q.; Gu, L.Q.; Ye, J.M.; et al. Rutaecarpine analogues reduce lipid accumulation in adipocytes via inhibiting adipogenesis/lipogenesis with AMPK activation and UPR suppression. ACS Chem. Biol. 2013, 8, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMP-activated protein kinase: A target for drugs both ancient and modern. Chem. Biol. 2012, 19, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Marín-Aguilar, F.; Pavillard, L.E.; Giampieri, F.; Bullón, P.; Cordero, M.D. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. Int. J. Mol. Sci. 2017, 18, 288. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabata, K.; Sawada, K.; Ikeda, K.; Fukuda, I.; Kawasaki, K.; Yamamoto, N.; Ashida, H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol. Nutr. Food Res. 2011, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, B.; Li, L.; Zhong, J. Effects of angelica keiskei chalcone on expression of glucose transporter proteins in liver and skeletal muscle cells of type 2 diabetic rats. Wei Sheng Yan Jiu 2013, 42, 107–109, 113. [Google Scholar] [PubMed]
- Muller, C.J.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ohnogi, H.; Kudo, Y.; Tahara, K.; Sugiyama, K.; Enoki, T.; Hayami, S.; Sagawa, H.; Tanimura, Y.; Aoi, W.; Naito, Y.; et al. Six new chalcones from Angelica keiskei inducing adiponectin production in 3T3-L1 adipocytes. Biosci. Biotechnol. Biochem. 2012, 76, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Bak, E.J.; Woo, G.H.; Kim, J.M.; Quan, Z.; Kim, J.M.; Yoon, H.K.; Cheon, S.H.; Yoon, G.; Yoo, Y.J.; et al. Licochalcone E has an antidiabetic effect. J. Nutr. Biochem. 2012, 23, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Kumar, M.; Rahuja, N.; Lal Srivastava, D.S.; Srivastava, A.K.; Maurya, R. Synthesis and antihyperglycemic activity of phenolic C-glycosides. Bioorg. Med. Chem. Lett. 2011, 21, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Ebrahim-Habibi, A.; Yaghmaei, P.; Parivar, K.; Larijani, B. Core structure of flavonoids precursor as an antihyperglycemic and antihyperlipidemic agent: An in vivo study in rats. Acta. Biochim. Pol. 2010, 57, 553–560. [Google Scholar] [PubMed]
- Semwal, D.K.; Rawat, U.; Semwal, R.; Singh, R.; Krishan, P.; Singh, M.; Singh, G.J. Chemical constituents from the leaves of Boehmeria rugulosa with antidiabetic and antimicrobial activities. J. Asian Nat. Prod. Res. 2009, 11, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kang, S.W.; Kim, J.L.; Sung, H.Y.; Kwun, I.S.; Kang, Y.H. Isoliquiritigenin entails blockade of TGF-beta1-SMAD signaling for retarding high glucose-induced mesangial matrix accumulation. J. Agric. Food Chem. 2010, 58, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Damazio, R.G.; Zanatta, A.P.; Cazarolli, L.H.; Mascarello, A.; Chiaradia, L.D.; Nunes, R.J.; Yunes, R.A.; Silva, F.R. Nitrochalcones: Potential in vivo insulin secretagogues. Biochimie 2009, 91, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Nakamura, H.; Hata, S.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 2009, 16, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Yoon, N.Y.; Kang, S.S.; Kim, Y.S.; Choi, J.S. Inhibitory activities of prenylated flavonoids from Sophora flavescens against aldose reductase and generation of advanced glycation endproducts. J. Pharm. Pharmacol. 2008, 60, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Song, D.G.; Lee, J.Y.; Pan, C.H.; Um, B.H.; Jung, S.H. Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts. Biol. Pharm. Bull. 2008, 31, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Govorko, D.; Logendra, S.; Wang, Y.; Esposito, D.; Komarnytsky, S.; Ribnicky, D.; Poulev, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1503–E1510. [Google Scholar] [CrossRef] [PubMed]
- Logendra, S.; Ribnicky, D.M.; Yang, H.; Poulev, A.; Ma, J.; Kennelly, E.J.; Raskin, I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry 2006, 67, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, M.; Tiwari, P.; Tripathi, B.K.; Srivastava, A.K.; Pratap, R. Synthesis and antihyperglycemic activity of chalcone based aryloxypropanolamines. Bioorg. Med. Chem. 2004, 12, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K.; Ueda, H.; Moriyasu, M. Aldose reductase inhibitors from the nature. Curr. Med. Chem. 2003, 10, 1353–1374. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Jung, S.H.; Ji, J.; Shin, K.H.; Keum, S.R. Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. J. Pharm. Pharmacol. 2001, 53, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, G.; Antolini, L.; Benvenuti, S.; Costantino, L. Structural bases for the inhibition of aldose reductase by phenolic compounds. Bioorg. Med. Chem. 2000, 8, 1151–1158. [Google Scholar] [CrossRef]
- Peck, F.B.; Mann, M. Effect of hesperidin methyl chalcone, vitamin P, on diabetic retinopathy. Am. J. Med. Sci. 1949, 217, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Itani, S.I.; Saha, A.K.; Kurowski, T.G.; Coffin, H.R.; Tornheim, K.; Ruderman, N.B. Glucose autoregulates its uptake in skeletal muscle: Involvement of AMP-activated protein kinase. Diabetes 2003, 52, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Gruzman, A.; Babai, G.; Sasson, S. Adenosine monophosphate-activated protein kinase (AMPK) as a new target for antidiabetic drugs: A review on metabolic, pharmacological and chemical considerations. Rev. Diabet. Stud. 2009, 6, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Winder, W.W.; Wilson, H.A.; Hardie, D.G.; Rasmussen, B.B.; Hutber, C.A.; Call, G.B.; Clayton, R.D.; Conley, L.M.; Yoon, S.; Zhou, B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J. Appl. Physiol. 1997, 82, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Almabrouk, T.A.; Ewart, M.A.; Salt, I.P.; Kennedy, S. Perivascular fat, AMP-activated protein kinase and vascular diseases. Br. J. Pharmacol. 2014, 171, 595–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, T.J.; Tsai, Y.H.; Liao, M.C.; Du, Y.C.; Lien, P.J.; Sun, C.C.; Chang, F.R.; Wu, Y.C. Anti-diabetic properties of non-polar Toona sinensis Roem extract prepared by supercritical-CO2 fluid. Food Chem. Toxicol. 2012, 50, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Beguinot, F.; Nigro, C. Measurement of glucose homeostasis in vivo: Glucose and insulin tolerance tests. Methods Mol. Biol. 2012, 933, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. Methods Mol. Biol. 2012, 933, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.S. Experimental Animals and Animal Experimental Techniques; China Press of Traditional Chinese Medicine: Beijing, China, 1997; pp. 165–166. [Google Scholar]








| Con | HF | HF + 5L | HF + 5H | HF + 6L | HF + 6H | HF + Pio | |
|---|---|---|---|---|---|---|---|
| (n = 13) | (n = 9) | (n = 10) | (n = 10) | (n = 8) | (n = 10) | (n = 6) | |
| Weight (g) | 30.8 ± 0.3 | 41.3 ± 1.1 a | 34.0 ± 0.3 c,d | 34.1 ± 0.5 c,d | 36.3 ± 1.1 a,d | 32.9 ± 0.9 d | 34.0 ± 1.0 d |
| Fas-Glu | 260.5 ± 10.6 | 316.7 ± 18.1 c | 191.4 ± 9.8 b,d | 221.9 ± 13.7 d | 236.1 ± 9.4 e | 235.2 ± 16.2 d | 245.5 ± 16.7 f |
| Insulin | 66.4 ± 6.6 | 127.3 ± 19.0 c | 51.6 ± 30.5 f | 48.1 ± 13.4 f | 60.0 ± 18.4 f | 35.8 ± 10.9 e | 41.9 ± 18.3 f |
| HOMA-IR | 39.4 ± 2.7 | 105. 7 ± 13.5 a | 27.8 ± 13.1 d | 25.9 ± 7.5 d | 35.3 ± 11.8 d | 23.2 ± 8.3 d | 25.6 ± 11.9 d |
| T-CHO | 79.3 ± 4.1 | 141.6 ± 10.1 a | 129.6 ± 8.6 a | 123.2 ± 8.3 a | 161.2 ± 9.2 a | 144.8 ± 5.8 a | 127.2 ± 8.4 b |
| TG | 68.3 ± 6.3 | 57.8 ± 5.5 | 70.7 ± 5.7 | 65.5 ± 4.1 | 62.3 ± 3.4 | 54.9 ± 3.0 | 55.7 ± 3.7 |
| GPT | 47.6 ± 2.9 | 46.7 ± 3.4 | 43.8 ± 3.7 | 42.0 ± 2.9 | 59.1 ± 5.2 | 46.3 ± 4.0 | 51.2 ± 4.3 |
| Crea | 0.25 ± 0.05 | 0.16 ± 0.02 | 0.18 ± 0.02 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-T.; Chang, F.-R.; Tsai, Y.-H.; Wu, Y.-C.; Hsieh, T.-J. 2-Bromo-4′-methoxychalcone and 2-Iodo-4′-methoxychalcone Prevent Progression of Hyperglycemia and Obesity via 5′-Adenosine-Monophosphate-Activated Protein Kinase in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 2763. https://doi.org/10.3390/ijms19092763
Hsieh C-T, Chang F-R, Tsai Y-H, Wu Y-C, Hsieh T-J. 2-Bromo-4′-methoxychalcone and 2-Iodo-4′-methoxychalcone Prevent Progression of Hyperglycemia and Obesity via 5′-Adenosine-Monophosphate-Activated Protein Kinase in Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2018; 19(9):2763. https://doi.org/10.3390/ijms19092763
Chicago/Turabian StyleHsieh, Chi-Ting, Fang-Rong Chang, Yi-Hong Tsai, Yang-Chang Wu, and Tusty-Jiuan Hsieh. 2018. "2-Bromo-4′-methoxychalcone and 2-Iodo-4′-methoxychalcone Prevent Progression of Hyperglycemia and Obesity via 5′-Adenosine-Monophosphate-Activated Protein Kinase in Diet-Induced Obese Mice" International Journal of Molecular Sciences 19, no. 9: 2763. https://doi.org/10.3390/ijms19092763