Reptiles in Space Missions: Results and Perspectives
Abstract
:1. Introduction
- (i)
- (ii)
- (iii)
- (iv)
- scanning Electron Microscopy (SEM) [45];
- (v)
- (vi)
2. Behavioral Adaptations of Geckos to Spaceflight Conditions
3. Gecko Brain in Prolonged Spaceflight Experiments
4. Reptile Internal Organs in the Spaceflight Experiments
4.1. Liver
4.2. Pancreas
4.3. Stomach and Small Intestine
4.4. Spleen
4.5. Heart
4.6. Lungs
5. Weightlessness Effects on the Skeletal Bone of Reptiles in the Prolonged Space Experiments
5.1. X-Ray Fluorescence Analysis (XFA), Scanning Electron Microscopy, and Tomographic Studies
5.2. Limb and Visceral Bones of Geckos After 12–30-Day Spaceflights
5.3. Caudal Vertebrae of Geckos after 12–30-Day Spaceflight
5.3.1. X-ray Fluorescence Analysis (XFA)
- (i)
- An increase in the signal from Ca and Sr for the samples of series Foton-M3.
- (ii)
- The absence of elements As, Pb, and Br in the composition of the samples of series Foton-M3.
- (iii)
- A decrease in the signal intensity from Cu in the samples of series Foton-M3.
5.3.2. Scanning Electron Microscopy
5.3.3. X-ray μCT
5.4. Cellular and Molecular Bone Studies after the Spaceflights
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ilyin, E.A.; Smirnov, I.A.; Soldatov, P.E.; Orlov, O.I. Gerbil experiment in the flight of spececraft “FOTON-M3”. Aviakosm. Ekolog. Med. 2009, 43, 21–25. [Google Scholar]
- Santucci, D.; Kawano, F.; Ohira, T.; Terada, M.; Nakai, N.; Francia, N.; Alleva, E.; Aloe, L.; Ochiai, T.; Cancedda, R.; et al. Evaluation of Gene, Protein and Neurotrophin Expression in the Brain of Mice Exposed to Space Environment for 91 Days. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Liu, Y.; Ruggiu, A.; Tavella, S.; Biticchi, R.; Santucci, D.; Schwartz, S.; Ciparelli, P.; Falcetti, G.; Tenconi, C.; et al. The mice drawer system (MDS) experiment and the space endurance record-breaking mice. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Andreev-Andrievskiy, A.; Popova, A.; Boyle, R.; Alberts, J.; Shenkman, B.; Vinogradova, O.; Dolgov, O.; Anokhin, K.; Tsvirkun, D.; Soldatov, P.; et al. Mice in Bion-M 1 space mission: Training and selection. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.E.; Moyer, E.L.; Talyansky, Y.; Lowe, M.; Padmanabhan, S.; Choi, S.; Gong, C.; Cadena, S.M.; Stodieck, L.; Globus, R.K. Behavior of mice aboard the International Space Station. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Khvatov, I.A.; Gulimova, V.I.; Barabanov, V.M.; Sokolov, A.Y.; Saveliev, S.V.; Kharitonov, A.N. Peculiar features of the adaptive behavior of thick-toed geckos in the orbital spaceflight experiment. Exp. Psychol. 2014, 7, 44–56. [Google Scholar]
- Von Baumgarten, R.J.; Simmonds, R.C.; Boyd, J.F.; Garriott, O.K. Effects of prolonged weightlessness on the swimming pattern of fish aboard Skylab 3. Aviat. Space Environ. Med. 1975, 46, 902–906. [Google Scholar] [PubMed]
- Ijiri, K. Fish Mating Experiment in Space-What It Aimed at and How It Was Prepared. Biol. Sci. Space 1995, 9, 3–16. [Google Scholar] [CrossRef]
- Ijiri, K.; Mizuno, R.; Eguchi, H. Use of an otolith-deficient mutant in studies of fish behavior in microgravity. Adv. Space Res. 2003, 32, 1501–1512. [Google Scholar] [CrossRef]
- Takabayashi, A.; Ohara, K.; Ohmura, T.; Watanabe, S.; Mori, S.; Tanaka, M.; Sakuragi, S. Mechanism of Vestibular Adaptation of Fish under Microgravity. Biol. Sci. Space 1997, 11, 351–354. [Google Scholar] [CrossRef]
- Rahmann, H.; Anken, R.H. Gravitational neurobiology of fish. Adv. Space. Res. 2000, 25, 1985–1995. [Google Scholar] [CrossRef]
- Anken, R.H. Neurophysiology of Developing Fish at Altered Gravity: Background—Facts–Perspectives. Adv. Space Biol. Med. 2003, 9, 173–200. [Google Scholar] [CrossRef]
- Snetkova, E.; Chelnaya, N.; Serova, L.; Saveliev, S.; Cherdanzova, E.; Pronych, S.; Wassersug, R. Effects of Space Flight on Xenopus laevis Larval Development. J. Exp. Zool. 1995, 273, 21–32. [Google Scholar] [CrossRef]
- Mitashov, V.; Brushlinskaya, N.; Grigoryan, E.; Tuchkova, S.Y.; Anton, H.J. Regeneration of organs and tissues in lower vertebrates during and after space flight. Adv. Space. Res. 1996, 17, 241–255. [Google Scholar] [CrossRef]
- Gualandris, L.; Grinfeld, S.; Foulquier, F.; Kan, P.; Duprat, A. Thepleurodele, an animal model for space biology studies. Adv. Space. Res. 1996, 17, 265–268. [Google Scholar] [CrossRef]
- Gualandris-Parisot, L.; Husson, D.; Foulquier, F.; Kan, P.; Davet, J.; Aimar, C.; Dournon, C.; Duprat, A.M. Pleurodeles waltl, amphibian, Urodele, is a suitable biological model for embryological and physiological space experiments on a vertebrate. Adv. Space. Res. 2001, 28, 569–578. [Google Scholar] [CrossRef]
- Grigoryan, E.N.; Mitashov, V.I.; Anton, H.J. Urodelean amphibians in studies on microgravity: Effects upon organ and tissue regeneration. Adv. Space. Res. 2002, 30, 757–764. [Google Scholar] [CrossRef]
- Frippiat, J.-P. Contribution of the urodele amphibian Pleurodeles waltl to the analysis of spaceflight-associated immune system deregulation. Mol. Immunol. 2013, 56, 434–441. [Google Scholar] [CrossRef]
- Meleshko, G.I.; Shepelev, E.I.; Gur’eva, T.S.; Bodia, K.; Sabo, V. The embryonic development of birds in weightlessness. Kosm. Biol. Aviakosm. Med. 1991, 25, 37–39. [Google Scholar]
- Boďa, K.; Sabo, V.; Juráni, M.; Guryeva, T.S.; Kočišová, J.; Košťál, Ľ.; Lauková, A.; Dadasheva, O.A. Embryonic Development and Behaviour of Japanese Quail Exposed to Microgravity. Acta Vet. Brno. 1992, 61, 99–107. [Google Scholar] [CrossRef]
- Dadasheva, O.A.; Gur’eva, T.S.; Sychev, V.N.; Jehns, G. Characteristics of morphogenesis of the Japanese quail embryos during microgravity. Aviakosm. Ekolog. Med. 1998, 32, 38–41. [Google Scholar]
- Orban, J.I.; Piert, S.J.; Guryeva, T.S.; Hester, P.Y. Calcium utilization by quail embryos during activities preceding space flight and during embryogenesis in microgravity aboard the orbital space station MIR. J. Gravit. Physiol. 1999, 6, 33–41. [Google Scholar]
- Barrett, J.E.; Wells, D.C.; Paulsen, A.Q.; Conrad, G.W. Embryonic quail eye development in microgravity. J. Appl. Physiol. 2000, 88, 1614–1622. [Google Scholar] [CrossRef] [Green Version]
- Lenhardt, L.; Cigankova, V.; Zibrin, M.; Kocisova, J.; Tomkova, I.; Sabo, V.; Boda, K.; Dadasheva, O.A.; Gurieva, T.S.; Mozes, S. Functional development of small intestine of Japanese quail hatched on MIR orbital station. Acta Vet. Brno. 2001, 70, 127–131. [Google Scholar] [CrossRef]
- Von Beckh, H.J.A. Experiments with animals and human subjects under sub- and zero-gravity conditions during the dive and parabolic flight. J. Aviat. Med. 1954, 25, 235–241. [Google Scholar]
- Wassersug, R.J.; Roberts, L.; Gimian, J.; Hughes, E.; Saunders, R.; Devison, D.; Woodbury, J.; O’Reilly, J.C. The behavioral responses of amphibians and reptiles to microgravity on parabolic flights. Zoology 2005, 108, 107–120. [Google Scholar] [CrossRef]
- Sutulov, L.S.; Kulkin, S.G.; Saxonov, J.L.; Sutulov, J.L.; Konnova, N.I.; Trushina, L.V.; Severgina, E.S.; Samsonova, L.L.; Sonina, S.N.; Selivanova, T.V.; et al. Post-flight histological analysis of turtles aboard Zond 7. Life Sci. Space Res. 1971, 9, 125–128. [Google Scholar]
- Stupakov, G.P.; Volozhin, A.; Korzhenyants, V.A.; Yagodovskii, V.S.; Polyakov, A.N.; Korolev, V.V.; Elivanov, V.A. Influence of long space flight factors on skeletal status in turtles. Pathol. Physiol. Exp. Therapy 1979, 6, 9–14. [Google Scholar]
- Gulimova, V.I.; Nikitin, V.B.; Asadchikov, V.E.; Buzmakov, A.V.; Okshtein, I.L.; Almeida, E.A.C.; Ilyin, E.A.; Tairbekov, M.G.; Saveliev, S.V. Effect of 16-day spaceflight on the morphology of thick-toed geckos (Pachydactylus turnery Gray, 1846). J. Gravit. Physiol. 2006, 13, 197–200. [Google Scholar]
- Almeida, E.A.C.; Roden, C.; Phillips, J.A.; Yusuf, R.; Globus, R.K.; Searby, N.; Vercoutere, W.; Morey-Holton, E.; Gulimova, V.; Saveliev, S.; et al. Development of the gecko (Pachydactylus turneri) animal model during Foton M-2 to study comparative effects of microgravity in terrestrial and aquatic organisms. J. Gravit. Physiol. 2006, 13, 193–196. [Google Scholar]
- Nikitin, V.B.; Gulimova, V.I.; Ilyin, E.A.; Asadchikov, V.E.; Buzmakov, A.V.; Okshtein, I.L.; Saveliev, S.V. Comparative analysis of the skeletal changes in tetrapods after brief influence of microgravity. J. Gravit. Physiol. 2007, 14, 103–104. [Google Scholar]
- Nikitin, V.B.; Proshchina, A.E.; Kharlamova, A.S.; Barabanov, V.M.; Krivova, J.S.; Godovalova, O.S.; Savelieva, E.S.; Makarov, A.N.; Gulimova, V.I.; Okshtein, I.L.; et al. Comparative studies of the thick-toed geckos after 16 and 12 days spaceflights in Foton-M experiments. J. Gravit. Physiol. 2008, 15, 285–288. [Google Scholar]
- Saveliev, S.V.; Gulimova, V.I.; Barabanov, V.M.; Proshchina, A.E.; Kurtova, A.I.; Krivova, Y.S.; Kharlamova, A.S.; Buzmakov, A.V.; Zolotov, D.A.; Senin, R.A.; et al. Study of thick-toed geckos and murine caudal vertebrae. In Space Scientific Project “BION-M1”: Medico-Biological Experiments and Investigations; Grigoriev, A.I., Ed.; SSC RF Institute for Biomedical Problems RAS: Moscow, Russian, 2016; pp. 298–306. ISBN 978-5-902119-32-6. [Google Scholar]
- Gulimova, V.I.; Barabanov, V.M.; Berdiev, R.K.; Proschina, A.E.; Kharlamova, A.S.; Saveliev, S.V. Species differences in the ability of geckos to adapt to the conditions of long-term orbital experiment onboard “BION-M1” and “FOTON-M4” biosatellites. Aviakosm. Ekolog. Med. 2016, 50, 55–57. [Google Scholar]
- Wassersug, R.; Izumi-Kurotani, A. The behavioral reactions of a snake and a turtle to abrupt decreases in gravity. Zoolog. Sci. 1993, 10, 505–509. [Google Scholar] [PubMed]
- Mori, S. Disorientation of animals in microgravity. J. Med. Sci. (Nagoya) 1995, 58, 71–81. [Google Scholar]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Puthoff, J.B.; Prowse, M.S.; Wilkinson, M.; Autumn, K. Changes in materials properties explain the effects of humidity on gecko adhesion. J. Exp. Biol. 2010, 213, 3699–3704. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.P.; Higham, T.E. A new angle on clinging in geckos: Incline, not substrate, triggers the deployment of the adhesive system. Proc. R. Soc. B. 2009, 276, 3705–3709. [Google Scholar] [CrossRef]
- Russell, A.P.; Oetelaar, G.S. Limb and digit orientation during vertical clinging in Bibron’s gecko, Chondrodactylus bibronii (A. Smith, 1846) and its bearing on the adhesive capabilities of geckos. Acta Zoologica 2016, 97, 345–360. [Google Scholar] [CrossRef]
- Barabanov, V.; Gulimova, V.; Berdiev, R.; Saveliev, S. Object play in thick-toed geckos during a space experiment. J. Ethol. 2015, 33, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Barabanov, V.; Gulimova, V.; Berdiev, R.; Saveliev, S. Attachment of thick-toed geckos in weightlessness and their reflex responses to flotation. Life Sci. Space Res. 2018, 18, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Proschina, A.E.; Kharlamova, A.S.; Barabanov, V.M.; Godovalova, O.S.; Gulimova, V.I.; Krivova, Y.S.; Makarov, A.N.; Nikitin, V.B.; Savelieva, E.S.; Saveliev, S.V. Effect of 12-day spaceflight on brain of thick-toed geckos. J. Grav. Physiol. 2008, 15, 297–298. [Google Scholar]
- Proshchina, A.E.; Kharlamova, A.S.; Barabanov, V.M.; Gulimova, V.I.; Saveliev, S.V. Vestibular cerebellum of thick-toed geckos (Chondrodactylus turnery Gray 1864) and C57/BL6N mice after long-term space flight on the biosatellite BION-M1. J. Chem. Neuroanat. 2017, 79, 58–65. [Google Scholar] [CrossRef]
- Asadchikov, V.E.; Senin, R.A.; Blagov, A.E.; Buzmakov, A.V.; Gulimova, V.I.; Zolotov, D.A.; Orekhov, A.S.; Osadchaya, A.S.; Podurets, K.M.; Savel’ev, S.V.; et al. Comparison of the Data of X-Ray Microtomography and Fluorescence Analysis in the Study of Bone-Tissue Structure. Crystall. Rep. 2012, 57, 700–707. [Google Scholar] [CrossRef]
- Buzmakov, A.; Chukalina, M.; Nikolaev, D.; Gulimova, V.; Saveliev, S.; Tereschenko, E.; Seregin, A.; Senin, R.; Zolotov, D.; Prun, V.; et al. Monochromatic Computed Microtomography using Laboratory and Synchrotron Sources and X-ray Fluorescence Analysis for Comprehensive Analysis of Structural Changes in Bone. J. Appl. Crystallogr. 2015, 48, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, G.M. The Genesis of Animal Play: Testing the Limits; MIT Press: Cambridge, UK, 2005. [Google Scholar]
- De Felipe, J.; Arellano, J.I.; Merchán-Pérez, A.; González-Albo, M.C.; Walton, K.; Llinás, R. Spaceflight Induces Changes in the Synaptic Circuitry of the Postnatal Developing Neocortex. Cereb. Cortex 2002, 12, 883–891. [Google Scholar] [CrossRef]
- Correia, M.J. Neuronal plasticity: Adaptation and readaptation to the environment of space. Brain Res. Rev. 1998, 28, 61–65. [Google Scholar] [CrossRef]
- Lee, J.K.; Koppelmans, V.; Riascos, R.F.; Hasan, K.M.; Pasternak, O.; Mulavara, A.P.; Bloomberg, J.J.; Seidler, R.D. Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 2019, 76, 412–419. [Google Scholar] [CrossRef]
- Van Ombergen, A.; Demertzi, A.; Tomilovskaya, E.; Jeurissen, B.; Sijbers, J.; Kozlovskaya, I.B.; Parizel, P.M.; van de Heyning, P.H.; Sunaert, S.; Laureys, S.; et al. The effect of spaceflight and microgravity on the human brain. J. Neurol. 2017, 264, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Clément, G.; Ngo-Anh, J.T. Space physiology II: Adaptation of the central nervous system to space flight--past, current, and future studies. Eur. J. Appl. Physiol. 2013, 113, 1655–1672. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364. [Google Scholar] [CrossRef]
- Pietsch, J.; Bauer, J.; Egli, M.; Infanger, M.; Wise, P.; Ulbrich, C.; Grimm, D. The effects of weightlessness on the human organism and mammalian cells. Curr. Mol. Med. 2011, 11, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Sarkar, S.; Ramesh, V.; Hayes, B.E.; Thomas, R.L.; Wilson, B.L.; Kim, H.; Barnes, S.; Kulkarni, A.; Pellis, N.; et al. Proteomic analysis of mice hippocampus in simulated microgravity environment. J. Proteome Res. 2006, 5, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Jandial, R.; Hoshide, R.; Waters, J.D.; Limoli, C.L. Space-brain: The negative effects of space exposure on the central nervous system. Surg. Neurol. Int. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Allen, P.L.; Birdsall, H.H. Effects of Space Flight on Mouse Liver versus Kidney: Gene Pathway Analyses. Int. J. Mol. Sci. 2018, 19, 4106. [Google Scholar] [CrossRef]
- Proshchina, A.E.; Besova, N.V.; Voronov, K.A.; Gulimova, V.I.; Serova, L.V.; Savel’ev, S.V. Morphogenesis of asymmetry of rat brain nuclei under normal conditions and during exposure to microgravitation. Bull. Exp. Biol. Med. 2000, 130, 908–911. [Google Scholar] [CrossRef]
- Mao, X.W.; Sandberg, L.B.; Gridley, D.S.; Herrmann, E.C.; Zhang, G.; Raghavan, R.; Zubarev, R.A.; Zhang, B.; Stodieck, L.S.; Ferguson, V.L.; et al. Proteomic Analysis of Mouse Brain Subjected to Spaceflight. Int. J. Mol. Sci. 2019, 20, 7. [Google Scholar] [CrossRef]
- Krasnov, I.B. Gravitational neuromorphology. Adv. Space Biol. Med. 1994, 4, 85–110. [Google Scholar] [CrossRef]
- Day, J.R.; Frank, A.T.; O’Callaghan, J.P.; DeHart, B.W. Effects of microgravity and bone morphogenetic protein II on GFAP in rat brain. J. Appl. Physiol. 1998, 85, 716–722. [Google Scholar] [CrossRef]
- Thornton, W.E.; Bonato, F. Space motion sickness and motion sickness: Symptoms and etiology. Aviat. Space Environ. Med. 2013, 84, 716–721. [Google Scholar] [CrossRef]
- Kalb, R.; Solomon, D. Space exploration, Mars, and the nervous system. Arch. Neurol. 2007, 64, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Bruce, L.L. Adaptations of the vestibular system to short and long-term exposures to altered gravity. Adv. Space Res. 2003, 32, 1533–1539. [Google Scholar] [CrossRef]
- Cohen, B.; Yakushin, S.B.; Holstein, G.R.; Dai, M.; Tomko, D.L.; Badakva, A.M.; Kozlovskaya, I.B. Vestibular experiments in space. Adv. Space Biol. Med. 2005, 10, 105–164. [Google Scholar] [PubMed]
- Holstein, G.R.; Kukielka, E.; Martinelli, G.P. Anatomical observations of the rat cerebellar nodulus after 24 hr of spaceflight. J. Gravit. Physiol. 1999, 6, 47–50. [Google Scholar]
- Krasnov, I.B. Electron microscopy analysis of the structural elements of the vestibular input to nodulus Purkinje’s cells in rats exposed to a 9-day space flight. Aviakosm. Ekolog. Med. 2008, 42, 20–27. [Google Scholar]
- Krasnov, I.B.; Krasnikov, G.V. Purkinje’s cells in the vestibular and proprioceptive segments of rat’s cerebellum following 14-day space flight. Aviakosm. Ekolog. Med. 2009, 43, 43–47. [Google Scholar]
- Kharlamova, A.S.; Proshchina, A.E.; Gulimova, V.I.; Barabanov, V.M.; Junnemann, O.A.; Saveliev, S.V. Gecko Cerebellum after a Long-Term Space Flight during the “BION-M1” Space Mission. In Development of the Cerebellum: Clinical and Molecular Perspectives; Fabbri, S., Ed.; NOVA Medicine & Health: New York, NY, USA, 2018; pp. 119–146. ISBN 978-1-53614-317-1. [Google Scholar]
- Sun, X.Q.; Xu, Z.P.; Zhang, S.; Cao, X.S.; Liu, T.S. Simulated weightlessness aggravates hypergravity-induced impairment of learning and memory and neuronal apoptosis in rats. Behav. Brain Res. 2009, 199, 197–202. [Google Scholar] [CrossRef]
- Mao, X.W.; Byrum, S.; Nishiyama, N.C.; Pecaut, M.J.; Sridharan, V.; Boerma, M.; Tackett, A.J.; Shiba, D.; Shirakawa, M.; Takahashi, S.; et al. Impact of Spaceflight and Artificial Gravity on the Mouse Retina: Biochemical and Proteomic Analysis. Int. J. Mol. Sci. 2018, 19, 2546. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kulikov, A.V.; Kondaurova, E.M.; Tsybko, A.S.; Kulikova, E.A.; Krasnov, I.B.; Shenkman, B.S.; Sychev, V.N.; Bazhenova, E.Y.; Sinyakova, N.A.; et al. Effect of actual long-term spaceflight on BDNF, TrkB, p75, BAX and BCL-XL genes expression in mouse brain regions. Neuroscience 2015, 284, 730–736. [Google Scholar] [CrossRef]
- Lazzari, M.; Franceschini, V. Glial fibrillary acid protein and vimentin immunoreactivity of astroglial cells in the central nervous system of adult Podarcis sicula (Squamata, Lacertidae). J. Anat. 2001, 198, 67–75. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Wang, D.; Zeng, F.; Wei, Y.; Wang, F.; Feng, C.; Li, N.; Dai, R.; Deng, Y.; et al. Effects of simulated microgravity on human brain nervous tissue. Neurosci. Lett. 2016, 627, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lukaszevicz, A.C.; Sampaio, N.; Guegan, C.; Benchoua, A.; Couriaud, C.; Chevalier, E.; Sola, B.; Lacombe, P.; Onteniente, B. High Sensitivity of Protoplasmic Cortical Astroglia to Focal Ischemia. J. Cereb. Blood Flow Metab. 2002, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Nishimura, M.; Kuwahara, Y.; Ueda, N.; Naitoh, S.; Kume, M.; Yamamoto, Y.; Fujita, J.; Funae, Y.; Fukumoto, M. Analysis of gene and protein expression of cytochrome P450 and stress-associated molecules in rat liver after spaceflight. Pathol. Int. 2008, 58, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Proshchina, A.E.; Krivova, Y.S.; Saveliev, S.C. Pancreas of C57 black mice after long-term space flight (Bion-M1 Space Mission). Life Sci. Space Res. 2015, 7, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Blaber, E.A.; Pecaut, M.J.; Jonscher, K.R. Spaceflight Activates Autophagy Programs and the Proteasome in Mouse Liver. Int. J. Mol. Sci. 2017, 18, 2062. [Google Scholar] [CrossRef]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of space flight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Izumi-Kurotani, A.; Mogami, Y.; Okuno, M.; Naitoh, T.; Wassersug, R.J. The Frogin Space (FRIS) experiment on board Space Station Mir: Final report and follow-on studies. Biol. Sci. Space 1997, 11, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.F.; Kita, K.; Ikuma, H.; Ohnaka, K.; Koike, S.; Takahashi, S.; Shiraishi, A.; Ohashi, S. Effects of gravity and oriental medicine, tochu (Eucommiaulmoides Oliver) leaves on tree Frog Hyla japonica. Biol. Sci. Space. 1991, 5, 202–207. [Google Scholar] [CrossRef]
- Pecaut, M.J.; Mao, X.W.; Bellinger, D.L.; Jonscher, K.R.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Mohney, R.P.; Gridley, D.S. Is spaceflight-induced immune dysfunction linked to systemic changes in metabolism? PLoS ONE 2017, 12, e0174174. [Google Scholar] [CrossRef] [PubMed]
- Shubich, M.G.; Goriacheva, L.L.; Dudetskii, V.I.; Lutsenko, N.M.; Mogil’naya, G.M. Histochemical study of the digestive organs of rats on board for the space flight of the satellite “Cosmos-690”. Kosm. Biol. Aviakosm. Med. 1978, 12, 41–46. [Google Scholar] [PubMed]
- Pashchenko, P.S.; Zakharova, I.V. Changes in the pancreas structure after exposure of the body to gravitational overloads. Morfologiia 2006, 129, 62–67. [Google Scholar] [PubMed]
- Afonin, B.V.; Nichiporuk, I.A.; Nesterov, M.A.; Pechenkina, R.A.; Goncharova, N.P.; Belousova, I.V. Results of studies of carbohydrate metabolism and ultrasonography of the pancreas in man after continuous anti-orthostatic hypokinesia. Aviakosm. Ekolog. Med. 1999, 33, 23–28. [Google Scholar] [PubMed]
- Smirnov, K.V.; Syrykh, G.D.; Legen’kov, V.I.; Goland-Ruvinova, L.G.; Medkova, I.L. Digestive system status after prolonged space flights. Kosm. Biol. Aviakosm Med. 1982, 16, 19–22. [Google Scholar] [PubMed]
- Afonin, B.V. Analysis of possible causes activation a stomach and pancreas excretory and incretory function after completion of space flight on the international space station. Fiziol. Cheloveka 2013, 39, 62–70. [Google Scholar] [PubMed]
- Leach, C.S. An overview of the endocrine and metabolic changes in manned space flight. Acta Astronaut. 1981, 8, 977–986. [Google Scholar] [CrossRef]
- Leach, C.S.; Altchuler, S.I.; Cintron-Trevino, N.M. The endocrine and metabolic responses to space flight. Med. Sci. Sports Exerc. 1983, 15, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Afonin, B.V.; Noskov, V.B.; Poliakov, V.V. The state of the digestive system organs during long space flight. Fiziol. Cheloveka 2003, 29, 53–57. [Google Scholar]
- Hughson, R.L.; Robertson, A.D.; Arbeille, P.; Shoemaker, J.K.; Rush, J.W.; Fraser, K.S.; Greaves, D.K. Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H628–H638. [Google Scholar] [CrossRef] [Green Version]
- Afonin, B.V. Dynamics of glycemic profile at the women in long-term antiorthostatic hypokinesia. Fiziol. Cheloveka 2016, 42, 88–95. [Google Scholar] [CrossRef]
- Macho, L.; Kvetnansky, R.; Fickova, M.; Popova, I.A.; Grigoriev, A. Effects of exposure to space flight on endocrine regulations in experimental animals. Endocr. Regul. 2001, 35, 101–114. [Google Scholar]
- Tobin, B.W. Insulin secretion and sensitivity in space flight: Diabetogenic effects. Nutrition 2002, 18, 842–848. [Google Scholar] [CrossRef]
- Gambara, G.; Salanova, M.; Ciciliot, S.; Furlan, S.; Gutsmann, M.; Schiffl, G.; Ungethuem, U.; Volpe, P.; Gunga, H.C.; Blottner, D. Microgravity-Induced Transcriptome Adaptation in Mouse Paraspinal longissimus dorsi Muscle Highlights Insulin Resistance-Linked Genes. Front. Physiol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Tascher, G.; Brioche, T.; Maes, P.; Chopard, A.; O’Gorman, D.; Gauquelin-Koch, G.; Blanc, S.; Bertile, F. Proteome-wide Adaptations of Mouse Skeletal Muscles during a Full Month in Space. J. Proteome Res. 2017, 16, 2623–2638. [Google Scholar] [CrossRef] [Green Version]
- Buono, S.; Odierna, G., II; Putti, R. Morphology of the pancreas of some species belonging to the genera Phelsuma and Gecko (family Gekkonidae): Evidence of apoptotic process during the seasonal cycle. Anat. Embryol. (Berl.) 2006, 211, 413–421. [Google Scholar] [CrossRef]
- Martinez, E.M.; Yoshida, M.C.; Candelario, T.L.; Hughes-Fulford, M. Spaceflight and simulated microgravity cause a significant reduction of key gene expression in early T-cell activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R480–R488. [Google Scholar] [CrossRef] [Green Version]
- Duncker, H.-R. Stammesgeschichteder Struktur- und Funktionsprinzipiender Wirbeltierlungen. Verh. Anat. Ges. 1981, 75, 279–303. [Google Scholar]
- Perry, S.F. Reptilian Lungs: Functional Anatomy and Evolution. Advances in Anatomy. Embryology and Cell Biology; Springer: New York, NY, USA, 1983; Volume 79, pp. 1–81. [Google Scholar]
- Perry, S.F.; Sander, M. Reconstructing the evolution of the respiratory apparatus in tetrapods. Respir. Physiol. Neurobiol. 2004, 144, 125–139. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Shachlamov, V.A. Capillaries; VEDI Publishing: Moscow, Russia, 2007; p. 288. ISBN 978-5-94624-024-6. [Google Scholar]
- Pfeiffer, C.J.; Yamashita, M.; Izumi-Kurotani, A.; Koike, H.; Asashima, M. Cytopathologic observations of the lung of adult newts (Cynopspyrrhogaster) on-board the space shuttle, Columbia, during the Second International Microgravity Laboratory experiments. J. Submicrosc. Cytol. Pathol. 1995, 27, 501–509. [Google Scholar]
- Prisk, K.G. The Lung in Space. Clin. Chest. Med. 2005, 26, 415–438. [Google Scholar] [CrossRef]
- Prisk, G.K. Microgravity and the respiratory system. Eur. Respir. J. 2014, 43, 1459–1471. [Google Scholar] [CrossRef]
- Tian, J.; Pecaut, M.J.; Slater, J.M.; Gridley, D.S. Spaceflight modulate sexpression of extracellular matrix, adhesion, and profibrotic molecules in mouse lung. J. Appl. Physiol. 2010, 108, 162–171. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Rudimov, E.G.; Andreeva, E.R.; Grigoriev, A.I. The ICAM-1 expression level determines the susceptibility of human endothelial cells to simulated microgravity. J. Cell Biochem. 2018, 119, 2875–2885. [Google Scholar] [CrossRef]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef]
- Giuliani, A.; Mazzoni, S.; Ruggiu, A.; Canciani, B.; Cancedda, R.; Tavella, S. High-Resolution X-Ray Tomography: A 3D Exploration Into the Skeletal Architecture in Mouse Models Submitted to Microgravity Constraints. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Clément, G. Fundamentals of Space Medicine, 2nd ed.; Microcosm Press: El Segundo and Springer: New York, NY, USA, 2011. [Google Scholar]
- Nagaraja, M.P.; Risin, D. The current state of bone loss research: Data from spaceflight and microgravity simulators. J. Cell. Biochem. 2013, 114, 1001–1008. [Google Scholar] [CrossRef]
- Berg-Johansen, B.; Liebenberg, E.C.; Li, A.; Macias, B.R.; Hargens, A.R.; Lotz, J.C. Spaceflight-induced bone loss alters failure mode and reduces bending strength in murine spinal segments. J. Orthop. Res. 2015, 34, 48–57. [Google Scholar] [CrossRef]
- Lang, T.; Van Loon, J.J.W.A.; Bloomfield, S.; Vico, L.; Chopard, A.; Rittweger, J.; Kyparos, A.; Blottner, D.; Vuori, I.; Gerzer, R.; et al. Towards human exploration of space: The THESEUS review series on muscle and bone research priorities. NPJ Microgravity 2017, 3. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Martinez, D.A.; Boudreaux, R.D.; Mantri, A.V. Microgravity Stress: Bone and Connective Tissue. Comp. Physiol. 2016, 6, 645–686. [Google Scholar] [CrossRef]
- Clément, G.R.; Bukley, A.P.; Paloski, W.H. Artificial gravity as a countermeasure for mitigating physiological deconditioning during long-duration space missions. Front. Sys. Neurosci. 2015, 9, 92. [Google Scholar] [CrossRef]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.H.; Thomas, T.; Rehaillia, M.; Alexandre, C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef]
- Lang, T.; LeBlanc, A.; Evans, H.; Lu, Y.; Genant, H.; Yu, A. Cortical and trabecular bone mineral loss from the spine and hip in long duration spaceflight. J. Bone Miner. Res. 2004, 19, 1006–1012. [Google Scholar] [CrossRef]
- Tavella, S.; Ruggiu, A.; Giuliani, A.; Brun, F.; Canciani, B.; Manescu, A.; Marozzi, K.; Cilli, M.; Costa, D.; Liu, Y.; et al. Bone turnover in wild type and pleiotrophin-transgenic mice housed for three months in the International Space Station (ISS). PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Blaber, E.A.; Dvorochkin, N.; Lee, C.; Alwood, J.S.; Yousuf, R.; Pianetta, P.; Globus, R.K.; Burns, B.P.; Almeida, E.A. Microgravity induces pelvic bone loss through osteoclasticactivity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Lloyd, S.A.; Morony, S.E.; Ferguson, V.L.; Simske, S.J.; Stodieck, L.S.; Warmington, K.S.; Livingston, E.W.; Lacey, D.L.; Kostenuik, P.J.; Bateman, T.A. Osteoprotegerin is an effective countermeasure for spaceflight-induced bone loss in mice. Bone 2015, 81, 562–572. [Google Scholar] [CrossRef]
- Gerbaix, M.; Gnyubkin, V.; Farlay, D.; Olivier, C.; Ammann, P.; Courbon, G.; Laroche, N.; Genthial, R.; Follet, H.; Peyrin, F.; et al. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci. Rep. 2017, 7, 2659. [Google Scholar] [CrossRef]
- Chatani, M.; Mantoku, A.; Takeyama, K.; Abduweli, D.; Sugamori, Y.; Aoki, K.; Ohya, K.; Suzuki, H.; Uchida, S.; Sakimura, T.; et al. Microgravity promotes osteoclast activity in medaka fish reared at the international space station. Sci. Rep. 2015, 5, 14172. [Google Scholar] [CrossRef] [Green Version]
- Chatani, M.; Morimoto, H.; Takeyama, K.; Mantoku, A.; Tanigawa, N.; Kubota, K.; Suzuki, H.; Uchida, S.; Tanigaki, F.; Shirakawa, M.; et al. Acute transcriptional up-regulation specific to osteoblasts/osteoclasts in medaka fish immediately after exposure to microgravity. Sci. Rep. 2016, 6, 39545. [Google Scholar] [CrossRef] [Green Version]
- Kashima, I.; Nishimura, K.; Okamoto, Y.; Kanno, M. Image Analysis of Bone Changes in Hyla japonica Exposed to Microgravity on the MIR Orbital Station. Biol. Sci. Space 1991, 5, 190–193. [Google Scholar] [CrossRef]
- Keune, J.A.; Branscum, A.J.; Iwaniec, U.T.; Turner, R.T. Effects of spaceflight on bone microarchitecture in the axial and appendicular skeleton in growing ovariectomized rats. Sci. Rep. 2015, 5, 18671. [Google Scholar] [CrossRef]
- Barou, O.; Valentin, D.; Vico, L.; Tirode, C.; Barbier, A.; Alexandre, C.; Lafage-Proust, M.H. High-resolution three-dimensional micro-computed tomography detects bone loss and changes in trabecular architecture early: Comparison with DEXA and bone histomorphometry in a rat model of disuse osteoporosis. Invest. Radiol. 2002, 37, 40–46. [Google Scholar] [CrossRef]
- Watts, N.B. Clinical utility of biochemical markers of bone remodeling. Clin. Chem. 1999, 45, 1359–1368. [Google Scholar]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; van den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Balemans, W.; Patel, N.; Ebeling, M.; van Hul, E.; Wuyts, W.; Lacza, C.; Dioszegi, M.; Dikkers, F.G.; Hildering, P.; Willems, P.J.; et al. Identification ofa 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 2002, 39, 91–97. [Google Scholar] [CrossRef]
- Chang, M.-K.; Kramer, I.; Huber, T.; Kinzel, B.; Guth-Gundel, S.; Leupin, O.; Kneissel, M. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc. Nat. Acad. Sci. USA 2014, 111, 5187–5195. [Google Scholar] [CrossRef]
- Marotti, G. The structure of bone tissues and the cellular control of their deposition. Ital. J. Anat. Embryol. 1996, 101, 25–31. [Google Scholar]
- Martin, R. Toward a unifying theory of bone remodeling. Bone 2000, 26, 1–7. [Google Scholar] [CrossRef]
- Baron, R.; Rawadi, G. Mini review: Targeting the Wnt/B-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007, 148, 2635–2643. [Google Scholar] [CrossRef]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.; Weber, P.; Feng, J.; Bonewald, L.; Kneissel, M. Osteocyte Wnt/B-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef]
- Spatz, J.; Fields, E.; Yu, E.; Pajevic, P.; Bouxsein, M.; Sibonga, J.; Zwart, S.; Smith, S. Serum sclerostin increases in healthy adult men during bed rest. J. Clin. Endocrinol. Metab. 2012, 97, E1736–E1740. [Google Scholar] [CrossRef]
- Gaudio, A.; Pennisi, P.; Bratengeier, C.; Torrisi, V.; Lindner, B.; Mangiafico, R.; Pulvirenti, I.; Hawa, G.; Tringali, G.; Fiore, C. Increased serum sclerostin levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J. Clin. Endocrinol. Metab. 2010, 95, 2248–2253. [Google Scholar] [CrossRef]
- Macias, B.R.; Swift, J.M.; Nilsson, M.I.; Hogan, H.A.; Bouse, S.D.; Bloomfield, S.A. Simulated resistance training, but not alendronate, increases cortical bone formation and suppresses sclerostin during disuse. J. Appl. Physiol. 2012, 112, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, A.; Sugiyama, T.; Prasad, J.; Zaman, G.; Gross, T.; Lanyon, L.; Price, J. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos. Int. 2012, 23, 1225–1234. [Google Scholar] [CrossRef]
- Spatz, J.M.; Ellman, R.; Cloutier, A.M.; Louis, L.; van Vliet, M.; Suva, L.J.; Dwyer, D.; Stolina, M.; Ke, H.Z.; Bouxsein, M.L. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J. Bone Miner. Res. 2013, 28, 865–874. [Google Scholar] [CrossRef]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin mediates bone response to mechanical unloading through antagonizing wnt/b-catenin signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef]
- Morse, A.; McDonald, M.; Kelly, N.; Melville, K.; Schindeler, A.; Kramer, I.; Kneissel, M.; van der Meulen, M.; Little, D. Mechanical load increases in bone formation via a sclerostin-independent pathway. J. Bone Miner. Res. 2014, 29, 2456–2467. [Google Scholar] [CrossRef]
- Bonewald, L.; Johnson, M. Osteocytes, mechanosensing and Wnt signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef] [Green Version]




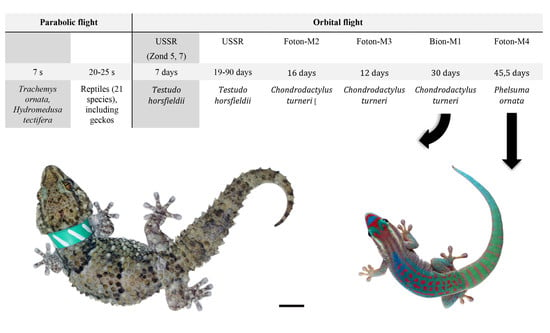
| Parabolic Flight | Orbital Flight | ||||||
|---|---|---|---|---|---|---|---|
| Zond 5, Zond 7 | Series of Orbital Flights (USSR) | Foton-M2 | Foton-M3 | Bion-M1 | Foton-M4 | ||
| 7 s | 20–25 s | 7 days | 19–90 days | 16 days | 12 days | 30 days | 45.5 days |
| Trachemys ornata, Hydromedusa tectifera [25] | Reptiles (21 species), including geckos [26] | Testudo horsfieldii [27] | Testudo horsfieldii [28] | Chondrodactylus turneri [29,30] | Chondrodactylus turneri [31,32] | Chondrodactylus turneri [33] | Phelsuma ornata [34] |
| Behavior | Behavior | Blood, internal organs, peripheral nervous system | Skeletal bones | Behavior, blood, internal organs, central nervous system, setae, skeletal bones, excrements | Behavior, internal organs, central nervous system, setae, skeletal bones, excrements | Behavior, internal organs, central nervous system, setae, skeletal bones | Behavior |
| Parameter | Foton-M2, 2005, 16-Day Flight | Foton-M3, 2007, 16-Day Flight | Bion-M1, 2013, 30-Day Flight | Foton-M4, 2014, 44.5-Day Flight |
|---|---|---|---|---|
| Species | Thick-toed geckos (Chondrodactylus turneri GRAY, 1864) | Thick-toed geckos (Chondrodactylus turneri GRAY, 1864) | Thick-toed geckos (Chondrodactylus turneri GRAY, 1864) | Thick-toed geckos (Phelsuma ornata GRAY, 1825) |
| Geckos in flight group | 5 (1 group from 5 geckos) | 5 (1 group from 5 geckos) | 15 (3 groups, each from 5 geckos) | 5 (1 group from 5 geckos) |
| Weight 1 (g) | F—16.9 *, M—24,7 * | 17.0 * | 20,2 * | F—3.8 *, M—4,3 * |
| Weight 1 (g) before flight/after flight | F—16.9/15.35 *, M—24.7/21.8 * | 17.0/15.6 * | 19.8/18.8 *. | F—3.8/2.3 *, M—4.3/3.0 * |
| Longevity in terrarium | 4–6 * years | |||
| Type of activity | Night | Day | ||
| Landing-fixation period (hours) | 32.0 | 13.5 | 13.5 | 6.1 |
| Temperature (C0) | 16.5 | 20.9 | 19.0 | 21.1 |
| Air regeneration | no | no | yes | no |
| Water supply | no | yes | yes | yes |
| Nutrition | no | no | yes | yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulimova, V.; Proshchina, A.; Kharlamova, A.; Krivova, Y.; Barabanov, V.; Berdiev, R.; Asadchikov, V.; Buzmakov, A.; Zolotov, D.; Saveliev, S. Reptiles in Space Missions: Results and Perspectives. Int. J. Mol. Sci. 2019, 20, 3019. https://doi.org/10.3390/ijms20123019
Gulimova V, Proshchina A, Kharlamova A, Krivova Y, Barabanov V, Berdiev R, Asadchikov V, Buzmakov A, Zolotov D, Saveliev S. Reptiles in Space Missions: Results and Perspectives. International Journal of Molecular Sciences. 2019; 20(12):3019. https://doi.org/10.3390/ijms20123019
Chicago/Turabian StyleGulimova, Victoria, Alexandra Proshchina, Anastasia Kharlamova, Yuliya Krivova, Valery Barabanov, Rustam Berdiev, Victor Asadchikov, Alexey Buzmakov, Denis Zolotov, and Sergey Saveliev. 2019. "Reptiles in Space Missions: Results and Perspectives" International Journal of Molecular Sciences 20, no. 12: 3019. https://doi.org/10.3390/ijms20123019