Published online Jun 21, 2006. doi: 10.3748/wjg.v12.i23.3746
Revised: September 28, 2005
Accepted: November 18, 2005
Published online: June 21, 2006
AIM: C-reactive protein (CRP) is an acute-phase reactant and a known indicator of the malignant potential of the tumour. The aim of this study was to investigate the significance of preoperative CRP as a parameter of the perioperative course and long-term prognosis in patients with squamous cell carcinoma and adenocarcinoma of the oesophagus.
METHODS: Serum CRP was determined preoperatively in 291 of 371 patients undergoing oesophagectomy for cancer from December 1989 to March 2004. Median patient age was 59 (28-79) year, 82.5% of patients were males. Squamous cell carcinoma was diagnosed in 151 (51.9%) and adenocarcinoma in 122 patients. Transhiatal oesophagectomy was done in 151 (51.9%) patients and 134 (46.0%) patients underwent the abdominothoracic procedure.
RESULTS: In 127 (43.6%) patients the preoperative serum CRP concentration was within the normal range (< 5 mg/dL), elevated CRP levels were measured in 164 (56.4%) patients. Tumour extension (P < 0.0005) and the number of lymph nodes affected by metastatic spread (P = 0.015) were significantly increased in the group with elevated CRP levels. Among the perioperative parameters both the number of blood transfusions (P = 0.006) and the general complication rate (P = 0.002) were higher in patients with elevated preoperative CRP levels. The long-term survival rate of 13.6 (0-109.8) mo was poorer in the group with elevated CRP levels compared to 18.9 (0-155.4) mo in the group with normal CRP levels (log-rank test: P = 0.107). Multivariate analysis with backward variables selection identified preoperative CRP as an independent prognostic factor of the long-term prognosis in patients with oesophageal carcinoma, with a hazard ratio of 1.182 (95% confidence interval: 1.030-1.356).
CONCLUSION: The preoperative serum CRP-level is an easily determined independent prognostic marker in patients with squamous cell carcinoma and adenocarcinoma of the oesophagus.
- Citation: Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol 2006; 12(23): 3746-3750
- URL: https://www.wjgnet.com/1007-9327/full/v12/i23/3746.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i23.3746
C-reactive protein (CRP) is an acute-phase reactant and a known indicator of the malignant potential of tumours. The synthesis is regulated by proinflammatory cytokines, which serve as growth factors in neoplastic processes[1]. These include in particular IL (interleukin)-1, IL-6, TNF (tumour necrosis factor)-α, IF (interferon)-γ, TGF (tumour growth factor)-β, EGF (epidermal growth factor) and LIF (leukaemia inhibiting factor). CRP is produced in the liver and electrophoresis shows a location of the protein between the beta- and gamma-globulin fractions.
The preoperative serum elevation of CRP has been identified as a significant prognostic factor in patients with colorectal cancer[2]. However, raised CRP concentrations as an indicator of a poorer prognosis in patients with oesophageal cancer have been demonstrated solely for squamous cell carcinoma[3,4]. The aim of this prospective study was to investigate the significance of C-reactive protein for the perioperative course and long-term prognosis in a patient population with histologic entities of both adenocarcinoma and squamous cell carcinoma of the oesophagus.
C-reactive protein was determined preoperatively in 291 of 371 patients undergoing oesophagectomy for carcinoma from December 1989 to March 2004. Median patient age was 59 (range from 28 to 79) year; 240 (82.5%) of the patients were males. Squamous cell carcinoma was diagnosed in 145 and adenocarcinoma in 122 patients. An undifferentiated carcinoma was found in 18 patients and 4 patients had other malignant tumours of the oesophagus. Patients receiving neoadjuvant chemoradiotherapy were excluded from the study.
Transhiatal oesophagectomy was carried out in 151 (51.9%) patients and 134 (46.0%) patients underwent abdominothoracic oesophagectomy with two-field lymphadenectomy (abdominal and mediastinal). Reconstruction was accomplished by pulled-up gastric tube in 264 of 285 patients (93.0%), by colon interposition in 12 (4.2%), and by small intestine interposition in 7 (2.5%) patients. The anatomic prevertebral oesophageal bed was used for the majority of these procedures. The remaining two patients did not undergo a primary reconstructive procedure.
Abdominothoracic oesophagectomy was routinely performed for squamous cell carcinoma. A transhiatal procedure was selected only for tumours with a distal location and malignancies without oesophageal wall penetration, or in the presence of a high general risk. Transhiatal oesophagectomy with abdominal and posterior mediastinal lymphadenectomy was carried out in adenocarcinomas, the two-field procedure was done in the presence of advanced tumour growth or extended lymph node involvement.
Data were collected prospectively in a specially established database and analysed retrospectively. In addition to routine demographic data, preoperative and intraoperative variables, tumour characteristics, as well as postoperative morbidity and mortality were documented. Long-term survival data were finally recorded in April 2004 and collected by telephone interview with the patients or their primary physicians.
Serum CRP was determined by latex-enhanced homoge-neous immunoassay[5] (wide-range) (Hitachi 917; Skill, Munich, Germany). In this assay, serum levels < 5 mg/dL are considered as normal values, those of ≥ 5 mL/dL are considered as indicators of existing pathology.
The SSPS 12.0 software package (SSPS, Chicago, IL, USA: 1999) and SAS 8.01 were used for statistical analysis. The patients were divided into two groups according to the C-reactive protein concentration measured preoperatively: those with CRP values in the normal range (< 5 mg/dL) and those with elevated CRP levels (≥ 5.0 mg/dL). Median values with ranges (minimum-maximum) as well as absolute (relative) frequencies are shown for both groups. The χ2-test with Pearson correction (χ2) and Fisher’s exact test (F) were used for the comparison of the categorical prognostic factors between the two groups. The t-test (t) was used for the comparison of continuous prognostic factors with symmetrical empirical distribution of the data. The Mann-Whitney U-test (U) served for the comparison of the other prognostic factors. The log-rank test (LR) was used for the comparison of survival times in the two groups. Morbidity, mortality, and long-term survival were assessed by multivariate analysis using Cox’s proportional hazards regression model (HR). The logarithm was found for CRP, which served as a continuous variable. Backward variables selection was carried out with the Wald test (W) to the level of α = 0.05.
Since no adjustment was made for the problem of multiple testing, all P values are descriptive.
Preoperative CRP concentrations in the normal range (< 5 mg/dL) were found in 127 (43.6%) patients, elevated CRP levels were found in 164 (56.4%) patients. There were no significant differences between the two groups in age, gender, and ASA-classification (P > 0.05). No differences were observed for tumour type and location, although tumour extension (in cm) (measured from surgical specimen fixed in formalin) (P < 0.0005), the number of dissected (P = 0.015) and positive lymph nodes (P = 0.015) were significantly higher in the group with elevated CRP levels. The distribution of pT-, pN-, and pM-categories was similar in both groups (P > 0.05) (Table 1).
| Parameter | CRP < 5.0 mg/dL | CRP ≥ 5.0 mg/dL | P-value |
| (n = 127) | (n = 164) | ||
| Histologic type (n = 289) | |||
| -squamous cell carcinoma | 56 (44%) | 89 (55%) | |
| -adenocarcinoma | 62 (49%) | 60 (37%) | 0.150 |
| -undifferentiated | 6 (5%) | 12 (7%) | |
| -other | 3 (2%) | 1 (1%) | |
| Location (n = 288) | |||
| -upper third | 9 (7%) | 15 (9%) | |
| -middle third | 42 (33%) | 49 (30%) | 0.748 |
| -lower third | 76 (60%) | 97 (60%) | |
| Tumour size (cm) (n = 281) | 3 (0.5-12) | 4.7 (0.7-20) | 0.0001 |
| Lymph nodes (n = 285) | |||
| -dissected | 23.5 (0-71) | 27 (2-86) | 0.015 |
| -positive | 1 (0-24) | 2 (0-41) | 0.015 |
| pT-category (n = 287) | |||
| -pT1 | 25 (20%) | 19 (12%) | |
| -pT2 | 34 (27%) | 31 (19%) | 0.055 |
| -pT3 | 61 (49%) | 98 (60%) | |
| -pT4 | 5 (4%) | 14 (9%) | |
| pN-category (n = 286) | |||
| -pN0 | 54 (43%) | 52 (32%) | |
| -pN1 | 69 (56%) | 107 (66%) | 0.062 |
| -pN2 | 1 (1%) | 3 (2%) | |
| pM-category (n = 283) | |||
| -pM0 | 96 (78%) | 116 (73%) | 0.177 |
| -pM1 | 27 (22%) | 44 (28%) |
Of the perioperative parameters, both the volume of blood transfusions (P = 0.006) and the number of internal medical complications (P = 0.002) were higher in patients with preoperative elevated CRP levels than in those with normal serum concentrations. No differences were found between the two patient groups in the length of the postoperative intensive care unit stay, the prevalence of surgical complications, or the morbidity and mortality rates (Table 2). Separate consideration of the two tumour entities showed that the squamous cell carcinoma was associated with a higher number of internal (P = 0.010) and surgical complications (P = 0.083) without a difference in the 30 d and hospital mortality rate, than adenocarcinoma where no significant differences were identified (P > 0.05). The comparison of the two groups showed that the preoperative CRP had no significant influence on the morbidity rate.
| Parameter | CRP < 5.0 mg/dL | CRP ≥ 5.0 mg/dL | P-value |
| (n = 127) | (n = 164) | ||
| Blood transfusion (n) | |||
| (n = 276) | 0 (0-22) | 1.5 (0-38) | 0.006 |
| ICU stay (d) | |||
| (n = 276) | 9.5 (2-107) | 11 (1-115) | 0.094 |
| Internal complications (n = 288) | |||
| 34 (27%) | 74 (45%) | 0.002 | |
| Surgical complications (n = 280) | 46 (36%) | 66 (41%) | 0.469 |
| -anastomotic leakage | 22 (18%) | 34 (21%) | 0.549 |
| -transplant necrosis | 3 (3%) | 7 (4%) | 0.522 |
| -mediastinitis | 2 (2%) | 3 (2%) | 0.999 |
| 30-d-mortality | |||
| (n = 286) | 9 (7%) | 12 (7%) | 0.999 |
| Hospital mortality | |||
| (n = 280) | 14 (11%) | 21 (13%) | 0.719 |
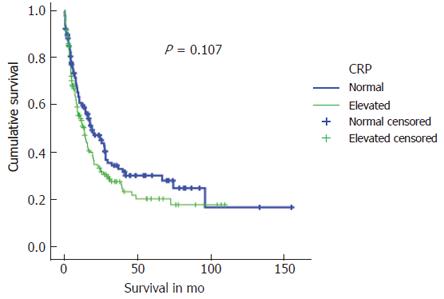
A median survival of 13.6 (0-109.8) mo in patients with preoperative elevated CRP levels was markedly poorer compared to 18.9 (0-155.4) in those with normal CRP levels (P = 0.107). Estimated long-term survival rates calculated by the Kaplan-Meier method for both patient groups are shown in Figure 1.
For multivariate analysis, a model for the prediction of survival time was established by carrying out a backward variables selection, comprising patient age, gender, BMI (body mass index), ASA classification, computed CRP logarithms, tumour type and location, R-classification, pT-, pN- and pM-category, as well as tumour size. The data analysis was only done on those 253 patients in whom all data were available. The analysis identified that the preoperative CRP with a hazard ratio of 1.182 (95% confidence interval: 1.030-1.356) was as an independent factor, which has an influence on the long-term prognosis in patients with oesophageal carcinoma (P = 0.0172).
Additional relevant prognostic factors included the pT-category (P < 0.0001), R-classification (P = 0.0113), and the surgical procedures in which transthoracic was more favourable with regard to long term-prognosis than transhiatal (P = 0.0345) (Table 3). The forward likelihood ratio variables selection yields a similar model. A separate analysis of each of the two histologic tumour types did not identify CRP as an independent prognostic factor (squamous cell carcinoma: P = 0.062; adenocarcinoma: P = 0.063). However, multivariate analysis showed that the surgical procedure (P = 0.0054) was the most significant prognostic indicator on survival in patients with squamous cell carcinoma, whereas the pT-category (P = 0.0002) was the most significant prognostic indicator for survival in patients with adenocarcinoma.
| Variable | 95% confidence interval (CI) | P-value | ||
| Hazard ratio | Lower border | Upper border | ||
| pT-category1 | < 0.0001 | |||
| -pT2 | 2.316 | 1.158 | 4.629 | 0.0174 |
| -pT3 | 4.240 | 2.211 | 8.131 | < 0.0001 |
| -pT4 | 4.432 | 1.854 | 10.596 | 0.0008 |
| R-classification2 | 0.0113 | |||
| 0 | 1.992 | 1.231 | 3.224 | 0.0050 |
| -R2 and R2 | -0.618 | 0.190 | 2.008 | 0.4234 |
| CRP (logarithm) | 1.182 | 1.030 | 1.356 | 0.0172 |
| Surgical approach3 | 0.0345 | |||
| -transthoracic | 0.680 | 0.486 | 0.953 | 0.0249 |
| -transhiatal | 0.326 | 0.079 | 1.338 | 0.1196 |
In the present study, patients with preoperatively elevated CRP concentrations had significantly larger tumours, a higher number of lymph nodes affected by metastatic spread with a higher total number of dissected lymph nodes. The correlation between acute-phase protein and biological behaviour in oesophageal cancer may be attributable to an inflammatory reaction, the endogenous response to tumour invasion[6,7].
The production of CRP is regulated by proinflamma-tory cytokines, in particular IL-6, IL-1 and TNFα, which serve as autocrine growth factors in neoplastic processes[2,8-11]. Acute-phase reactions of the organism have further been observed in tumour progression and tumour recurrence[12]. A correlation has been established between elevated serum CRP concentrations and malnutrition as well as impaired immunity in patients with oesophageal cancer[1].
While acute bacterial infections and collagen diseases are associated with a marked serum CRP elevation (> 10 mg/dL) at a latency period of 8-12 h for infections, only moderately elevated serum levels (< 10 mg/dL) have been observed in patients with malignomas[6,13].
Nozoe et al first presented immunohistochemical evidence of CRP expression in tumour cells of patients with squamous cell carcinoma of the oesophagus. The authors found that elevated serum levels of the protein, in addition the synthesis by hepatocytes as a response to the tumour, may be at least in part due to the production of CRP by the tumour itself[4]. In their study, the immunohistochemical expression of CRP in the tumour correlated with preoperative serum CRP levels. There were, however, no significant differences in the clinicopathologic characteristics, in particular the TNM-classification, between the patients with and without CRP expression of the tumour[4].
Comparable data on the adenocarcinoma have not been published to date.
Mortality rates after oesophagectomy cited in the literature range from 2%-25%[14]. These rates are in particular accounted by the development of pulmonary complications[15-17]. Various risk analyses have further described the impaired function of heart, liver and respiratory system, in addition to patient age and general status as independent predictors of perioperative morbidity and mortality[14,18-20]. A preoperative marker for the assessment of the intraoperative and postoperative course has not yet been established.
There was an increase in the required transfusion of packed erythrocytes in our patients with preoperatively elevated CRP, which may be due to the presence of larger tumours leading to extended operative times in this group. Furthermore, there were a greater number of general complications, especially those of pulmonary origin, in patients with a preoperative CRP value of ≥ 5.0 mg/dL. With a view to the described cytokine interaction, Katsuta et al found a close association between preoperative TNF-α and IL-1β-production, and pulmonary complications after transthoracic oesophagectomy[21]. In their study, however, they did not find that either preoperatively determined CRP levels or IL-6 had an influence on the development of postoperative pneumonia[21]. A limitation of the assessment of the importance of C-reactive protein in our patient population is that the abdominothoracic procedure, which is associated with a higher pulmonary morbidity rate, was selected more frequently (80/134 patients) than the transhiatal approach (80/151 patients) in the group with elevated CRP concentrations. The difference in the occurrence of general complications was therefore of relevance only in patients with squamous cell carcinoma, but not in those with adenocarcinoma.
In addition to the pT-category as the most significant pro-gnostic factor, R-classification and the surgical procedure, preoperatively determined CRP was an independent prognostic factor in our patient population after surgical treatment for oesophageal carcinoma.
A number of different molecular biologic analyses have established a correlation between the expression of epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), cyclin D, int-2, hst-1, p53-mutation, CD44-variants, MIB-1-proliferation and carcinogenesis in oesophageal carcinoma and its prognosis[22-28].
The importance of CRP as a prognostic indicator in oesophageal carcinoma has recently been described in patients with squamous cell carcinoma only[3,4,29], as well as by a study consisting to 6.5% of patients with adenocarcinoma[1]. Thus far there have been no studies conducted to investigate the role of CRP in patients with adenocarcinoma, or in patients comprising with both adenocarcinoma and squamous cell carcinoma. A search of the available literature demonstrates that this study is the first to identify preoperative CRP as an independent prognostic factor for long-term survival in a mixed patient population. The number of patients with squamous cell carcinoma was only slightly higher than that with adenocarcinoma (145/291 vs 122/291, respectively).
In summary, the results of this study show that preoperative CRP is an easily determined and independent prognostic marker in patients with squamous cell carcinoma and adenocarcinoma of the oesophagus. The significance of the role of this marker needs to be assessed in relation to that of other important prognostic factors of long-term survival, i.e. the pT-category and R-classification. However, the pT-category and R-classification become apparent only postoperatively, the assessment of CRP is a useful tool in the evaluation of the course prior to surgical intervention.
S- Editor Wang J L- Editor Zhao JB E- Editor Ma WH
| 1. | Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Aoki T, Sugaya M, Miyazawa Y, Hayashi H, Miyazaki S. Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2003;83:248-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Nozoe T, Korenaga D, Futatsugi M, Saeki H, Maehara Y, Sugimachi K. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus - significance as a tumor marker. Cancer Lett. 2003;192:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Senju O, Takagi Y, Uzawa R, Iwasaki Y, Suzuki T, Gomi K, Ishii T. A new immuno quantitative method by latex agglutination--application for the determination of serum C-reactive protein (CRP) and its clinical significance. J Clin Lab Immunol. 1986;19:99-103. [PubMed] [Cited in This Article: ] |
| 6. | Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313-336. [PubMed] [Cited in This Article: ] |
| 7. | Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 254] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Shimada H, Nabeya Y, Okazumi S, Matsubara H, Miyazawa Y, Shihratori T, Hayashi H, Aoki T, Sugaya M, Gunji Y. Prognostic value of preoperative serum immunosuppressive acidic protein in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2003;16:102-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 512] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Gelin J, Moldawer LL, Lönnroth C, Sherry B, Chizzonite R, Lundholm K. Role of endogenous tumor necrosis factor alpha and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991;51:415-421. [PubMed] [Cited in This Article: ] |
| 11. | Miki S, Iwano M, Miki Y, Yamamoto M, Tang B, Yokokawa K, Sonoda T, Hirano T, Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989;250:607-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 323] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Cooper EH, Stone J. Acute phase reactant proteins in cancer. Adv Cancer Res. 1979;30:1-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Gurleyik E, Gurleyik G, Unalmişer S. Accuracy of serum C-reactive protein measurements in diagnosis of acute appendicitis compared with surgeon's clinical impression. Dis Colon Rectum. 1995;38:1270-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Ferguson MK, Martin TR, Reeder LB, Olak J. Mortality after esophagectomy: risk factor analysis. World J Surg. 1997;21:599-603; discussion 603-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Whooley BP, Law S, Murthy SC, Alexandrou A, Wong J. Analysis of reduced death and complication rates after esophageal resection. Ann Surg. 2001;233:338-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Fagevik Olsén M, Wennberg E, Johnsson E, Josefson K, Lönroth H, Lundell L. Randomized clinical study of the prevention of pulmonary complications after thoracoabdominal resection by two different breathing techniques. Br J Surg. 2002;89:1228-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Fan ST, Lau WY, Yip WC, Poon GP, Yeung C, Lam WK, Wong KK. Prediction of postoperative pulmonary complications in oesophagogastric cancer surgery. Br J Surg. 1987;74:408-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Law SY, Fok M, Wong J. Risk analysis in resection of squamous cell carcinoma of the esophagus. World J Surg. 1994;18:339-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Lund O, Kimose HH, Aagaard MT, Hasenkam JM, Erlandsen M. Risk stratification and long-term results after surgical treatment of carcinomas of the thoracic esophagus and cardia. A 25-year retrospective study. J Thorac Cardiovasc Surg. 1990;99:200-209. [PubMed] [Cited in This Article: ] |
| 20. | Bartels H, Stein HJ, Siewert JR. Preoperative risk analysis and postoperative mortality of oesophagectomy for resectable oesophageal cancer. Br J Surg. 1998;85:840-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Katsuta T, Saito T, Shigemitsu Y, Kinoshita T, Shiraishi N, Kitano S. Relation between tumour necrosis factor alpha and interleukin 1beta producing capacity of peripheral monocytes and pulmonary complications following oesophagectomy. Br J Surg. 1998;85:548-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Tsuda T, Tahara E, Kajiyama G, Sakamoto H, Terada M, Sugimura T. High incidence of coamplification of hst-1 and int-2 genes in human esophageal carcinomas. Cancer Res. 1989;49:5505-5508. [PubMed] [Cited in This Article: ] |
| 23. | Lu SH, Hsieh LL, Luo FC, Weinstein IB. Amplification of the EGF receptor and c-myc genes in human esophageal cancers. Int J Cancer. 1988;42:502-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 110] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Jiang W, Kahn SM, Tomita N, Zhang YJ, Lu SH, Weinstein IB. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992;52:2980-2983. [PubMed] [Cited in This Article: ] |
| 25. | Kobayashi S, Koide Y, Endo M, Isono K, Ochiai T. The p53 gene mutation is of prognostic value in esophageal squamous cell carcinoma patients in unified stages of curability. Am J Surg. 1999;177:497-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Gotoda T, Matsumura Y, Kondo H, Ono H, Kanamoto A, Kato H, Watanabe H, Tachimori Y, Nakanishi Y, Kakizoe T. Expression of CD44 variants and prognosis in oesophageal squamous cell carcinoma. Gut. 2000;46:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Uchida S, Shimada Y, Watanabe G, Tanaka H, Shibagaki I, Miyahara T, Ishigami S, Imamura M. In oesophageal squamous cell carcinoma vascular endothelial growth factor is associated with p53 mutation, advanced stage and poor prognosis. Br J Cancer. 1998;77:1704-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Youssef EM, Matsuda T, Takada N, Osugi H, Higashino M, Kinoshita H, Watanabe T, Katsura Y, Wanibuchi H, Fukushima S. Prognostic significance of the MIB-1 proliferation index for patients with squamous cell carcinoma of the esophagus. Cancer. 1995;76:358-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Ikeda M, Natsugoe S, Ueno S, Baba M, Aikou T. Significant host- and tumor-related factors for predicting prognosis in patients with esophageal carcinoma. Ann Surg. 2003;238:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |