Abstract
Purpose
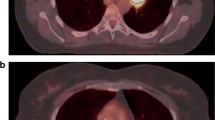
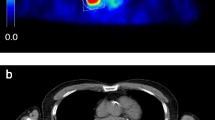
Assessment of the metabolically active tumour tissue by FDG PET is evolving for use in the diagnosis of non-small-cell lung cancer (NSCLC), in the planning of radiotherapy, and in follow-up and response evaluation. For exact evaluation accurate registration of PET and CT data is required. The registration process is usually based on rigid algorithms; however, nonrigid algorithms are increasingly being used. The influence of the registration method on FDG PET-based standardized uptake value (SUVmax) and metabolic tumour volume (MTV) definition has not yet been evaluated. We compared intra- and interindividual differences in SUV and MTV between rigid- and nonrigid-registered PET and CT acquired during different breathing manoeuvres.
Methods
The study group comprised 28 radiotherapy candidates with histologically proven NSCLC who underwent FDG PET acquisition and three CT acquisitions (expiration – EXP, inspiration – INS, mid-breath-hold – MID). All scans were registered with both a rigid (R) and a nonrigid (NR) procedure resulting in six fused datasets: R-INS, R-EXP, R-MID, NR-INS, NR-EXP and NR-MID. For the delineation of MTVs a contrast-oriented contouring algorithm developed in-house was used. To accelerate the delineation a semiautomatic software prototype was utilized.
Results
Tumour mean SUVmax did not differ for R and NR registration (R 17.5 ± 7, NR 17.4 ± 7; p=0.2). The mean MTV was higher by 3 ± 12 ml (p=0.02) in the NR group than in the R group, as was the mean tumour diameter (by 0.1 ± 0.2 cm; p<0.01). With respect to the three different breathing manoeuvres, there were no differences in MTV in the R group (p > 0.7). In intraindividual comparison there were no significant differences in MTVs concerning the registration pairs R-EXP (68 ± 88 ml) vs. NR-EXP (69 ± 85 ml) und R-MID (68 ± 86 ml) vs. NR-MID (69 ± 83 ml) (both p > 0.4). However, the MTVs were larger after NR registration during inspiration (R-INS 68 ± 82 vs. NR-INS 78 ± 93 ml; p=0.02).
Conclusion
The use of nonrigid algorithms may lead to a change in MTV, whose extent is influenced by the breathing manoeuvre on CT. Nonrigid registration methods cannot be recommended for the definition of MTV if the CT scan is performed during inspiration. The choice of registration algorithm has no significant impact on SUVmax.


Similar content being viewed by others
References
Grgic A, Yuksel Y, Groschel A, Schafers HJ, Sybrecht GW, Kirsch CM, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18)F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Med Mol Imaging. 2010;37:1087–94
Hellwig D, Baum RP, Kirsch C. FDG-PET, PET/CT and conventional nuclear medicine procedures in the evaluation of lung cancer: a systematic review. Nuklearmedizin. 2009;48:59–69, quiz N8–9
Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med. 2007;48 Suppl 1:78S–88S.
Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess. 2007;11:iii–iv, xi–267.
Nehmeh SA, Erdi YE. Respiratory motion in positron emission tomography/computed tomography: a review. Semin Nucl Med. 2008;38:167–76.
Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66.
Pietrzyk U. Does PET/CT render software registration obsolete? Nuklearmedizin. 2005;44 Suppl 1:S13–7.
Gilman MD, Fischman AJ, Krishnasetty V, Halpern EF, Aquino SL. Hybrid PET/CT of the thorax: when is computer registration necessary? J Comput Assist Tomogr. 2007;31:395–401.
Grgic A, Nestle U, Schaefer-Schuler A, Kremp S, Kirsch CM, Hellwig D. FDG-PET-based radiotherapy planning in lung cancer: optimum breathing protocol and patient positioning – an intraindividual comparison. Int J Radiat Oncol Biol Phys. 2009;73:103–11.
Goerres GW, Kamel E, Heidelberg TN, Schwitter MR, Burger C, von Schulthess GK. PET-CT image co-registration in the thorax: influence of respiration. Eur J Nucl Med Mol Imaging. 2002;29:351–60.
Krishnasetty V, Fischman AJ, Halpern EL, Aquino SL. Comparison of alignment of computer-registered data sets: combined PET/CT versus independent PET and CT of the thorax. Radiology. 2005;237:635–9.
Slomka PJ, Baum RP. Multimodality image registration with software: state-of-the-art. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S44–55.
Ireland RH, Dyker KE, Barber DC, Wood SM, Hanney MB, Tindale WB, et al. Nonrigid image registration for head and neck cancer radiotherapy treatment planning with PET/CT. Int J Radiat Oncol Biol Phys. 2007;68:952–7.
Grgic A, Nestle U, Schaefer-Schuler A, Kremp S, Ballek E, Fleckenstein J, et al. Nonrigid versus rigid registration of thoracic 18F-FDG PET and CT in patients with lung cancer: an intraindividual comparison of different breathing maneuvers. J Nucl Med. 2009;50:1921–6.
Lucignani G. Monitoring cancer therapy with PET: probably effective, but more research is needed. Eur J Nucl Med Mol Imaging. 2009;36:1520–5.
Daou D. Respiratory motion handling is mandatory to accomplish the high-resolution PET destiny. Eur J Nucl Med Mol Imaging. 2008;35:1961–70.
Kuhnigk JM, Dicken V, Zidowitz S, Bornemann L, Kuemmerlen B, Krass S, et al. Informatics in radiology (infoRAD): new tools for computer assistance in thoracic CT. Part 1. Functional analysis of lungs, lung lobes, and bronchopulmonary segments. Radiographics. 2005;25:525–36.
Slomka PJ, Dey D, Przetak C, Aladl UE, Baum RP. Automated 3-dimensional registration of stand-alone (18)F-FDG whole-body PET with CT. J Nucl Med. 2003;44:1156–67.
Fitton I, Steenbakkers RJ, Gilhuijs K, Duppen JC, Nowak PJ, van Herk M, et al. Impact of anatomical location on value of CT-PET co-registration for delineation of lung tumors. Int J Radiat Oncol Biol Phys. 2008;70:1403–7.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50 Suppl 1:11S–20S.
Schaefer A, Kremp S, Hellwig D, Rube C, Kirsch CM, Nestle U. A contrast-oriented algorithm for FDG-PET-based delineation of tumour volumes for the radiotherapy of lung cancer: derivation from phantom measurements and validation in patient data. Eur J Nucl Med Mol Imaging. 2008;35:1989–99.
Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302.
Weber WA. Assessing tumor response to therapy. J Nucl Med. 2009;50 Suppl 1:1S–10S.
Gilman MD, Fischman AJ, Krishnasetty V, Halpern EF, Aquino SL. Optimal CT breathing protocol for combined thoracic PET/CT. AJR Am J Roentgenol. 2006;187:1357–60.
Moreno A, Chambon S, Santhanam AP, Rolland JP, Angelini E, Bloch I. Combining a breathing model and tumor-specific rigidity constraints for registration of CT-PET thoracic data. Comput Aided Surg. 2008;13:281–98.
Nestle U, Schaefer-Schuler A, Kremp S, Groeschel A, Hellwig D, Rube C, et al. Target volume definition for 18F-FDG PET-positive lymph nodes in radiotherapy of patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:453–62.
Tylski P, Stute S, Grotus N, Doyeux K, Hapdey S, Gardin I, et al. Comparative assessment of methods for estimating tumor volume and standardized uptake value in (18)F-FDG PET. J Nucl Med. 2010;51:268–76
Liu HH, Balter P, Tutt T, Choi B, Zhang J, Wang C, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:531–40.
Gietema HA, Schaefer-Prokop CM, Mali WP, Groenewegen G, Prokop M. Pulmonary nodules: interscan variability of semiautomated volume measurements with multisection CT – influence of inspiration level, nodule size, and segmentation performance. Radiology. 2007;245:888–94.
Erdi YE, Nehmeh SA, Pan T, Pevsner A, Rosenzweig KE, Mageras G, et al. The CT motion quantitation of lung lesions and its impact on PET-measured SUVs. J Nucl Med. 2004;45:1287–92.
Nehmeh SA, Erdi YE, Pan T, Pevsner A, Rosenzweig KE, Yorke E, et al. Four-dimensional (4D) PET/CT imaging of the thorax. Med Phys. 2004;31:3179–86.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50 Suppl 1:31S–42S.
Liu C, Pierce 2nd LA, Alessio AM, Kinahan PE. The impact of respiratory motion on tumor quantification and delineation in static PET/CT imaging. Phys Med Biol. 2009;54:7345–62.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below are the links to the electronic supplementary material.
Supplementary Figure 1
a Correlation (r = 0.99, p < 0.001) between the tumour volumes (millilitres) in all patients measured in the rigid group (R-VOLUME) and the nonrigid group (NR-VOLUME), logarithmic scale. b Correlation (r = 0.99, p < 0.001) between the tumour diameters (centimetres) in all patients measured in the rigid group (R-DIAMETER) and nonrigid group (NR-DIAMETER), logarithmic scale. c Correlation (r = 0.99, p < 0.001) between the tumour SUVs in all patients measured in the rigid group (R-SUV) and nonrigid group (NR-SUV), logarithmic scale. Solid lines indicate the best fit. Dashed lines indicate lines of identity (DOC 2205 kb)
Supplementary Figure 2
a Correlation (r = 0.98, p < 0.001) between the tumour volumes (millilitres) during inspiration measured in the rigid group (R-INSP-VOLUME) and the nonrigid group (NR-INSP-VOLUME), logarithmic scale. Note the systematic overestimation of volumes after nonrigid registration. b Correlation (r = 0.99, p < 0.001) between the tumour volumes during expiration measured in the rigid group (R-EXP-VOLUME) and nonrigid group (NR-EXP-VOLUME), logarithmic scale. c Correlation (r = 0.99, p < 0.001) between the tumour volumes (millilitres) during mid-breath-hold measured in the rigid group (R-MID-VOLUME) and nonrigid group (NR-MID-VOLUME), logarithmic scale. Solid lines indicate the best fit. Dashed lines indicate lines of identity [(DOC 2198 kb)
Supplementary Figure 3
a Correlation (r = 0.98, p < 0.001) between SUVmax of the tumour lesions during inspiration measured in the rigid group (R -INSP-SUV) and nonrigid group (NR -INSP-SUV), logarithmic scale. b Correlation (r = 0.99, p < 0.001) between SUVmax of the tumour lesions during expiration measured in the rigid group (R -EXP-SUV) and nonrigid group (NR -EXP-SUV), logarithmic scale. c Correlation (r = 0.99, p < 0.001) between SUVmax of the tumour lesions during mid-breath-hold measured in the rigid group (R -MID-SUV) and nonrigid group (NR -MID-SUV), logarithmic scale. Solid lines indicate the best fit. Dashed lines indicate lines of identity (DOC 1480 kb)
Supplementary Table
SUVmean values in relation to breathing protocol used (DOC 28 kb)
Rights and permissions
About this article
Cite this article
Grgic, A., Ballek, E., Fleckenstein, J. et al. Impact of rigid and nonrigid registration on the determination of 18F-FDG PET-based tumour volume and standardized uptake value in patients with lung cancer. Eur J Nucl Med Mol Imaging 38, 856–864 (2011). https://doi.org/10.1007/s00259-010-1719-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1719-3