Abstract
Objectives
Chang’s method, the most widely used attenuation correction (AC) in brain single-photon emission computed tomography (SPECT), requires delineation of the outer contour of the head. Manual and automatic threshold-based methods are prone to errors due to variability of tracer uptake in the scalp. The present study proposes a new method for fully automated delineation of the head based on stereotactical normalization. The method was validated for SPECT with I-123-ioflupane.
Methods
The new method was compared to threshold-based delineation in 62 unselected patients who had received I-123-ioflupane SPECT at one of 3 centres. The impact on diagnostic power was tested for semi-quantitative analysis and visual reading of the SPECT images (six independent readers).
Results
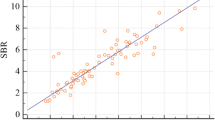
The two delineation methods produced highly consistent semi-quantitative results. This was confirmed by receiver operating characteristic analyses in which the putamen specific-to-background ratio achieved highest area under the curve with negligible effect of the delineation method: 0.935 versus 0.938 for stereotactical normalization and threshold-based delineation, respectively. Visual interpretation of DVR images was also not affected by the delineation method.
Conclusions
Delineation of the head contour by stereotactical normalization appears useful for Chang AC in I-123-ioflupane SPECT. It is robust and does not require user interaction.
Key Points
•Chang attenuation correction in brain SPECT requires delineation of the head contour.
•Manual and threshold-based methods are prone to errors.
•The study proposes a fully-automated method for delineation based on stereotactical normalization.
•The method is shown to work reliably in I-123-ioflupane SPECT.
•It might improve the workflow of I-123-ioflupane SPECT in everyday patient care.






Similar content being viewed by others
References
Bartenstein P, Grunwald F, Kuwert T et al (2000) Clinical applications of single photon emission tomography in neuromedicine. 1. Neuro-oncology, epilepsy, movement disorders, cerebrovascular disease. Nuklearmedizin 39:180–195
Booij J, Speelman JD, Horstink MW, Wolters EC (2001) The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 28:266–272
Booij J, Tissingh G, Boer GJ et al (1997) [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 62:133–140
Hesse S, Oehlwein C, Meyer PT et al (2003) Is there a role for I-123-FP-CIT SPECT in the management of suspected Parkinson's disease? J Nucl Med 44:234p–235p
Innis RB, Seibyl JP, Scanley BE et al (1993) Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci U S A 90:11965–11969
Seibyl JP, Marek KL, Quinlan D et al (1995) Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol 38:589–598
Tatsch K, Poepperl G (2013) Nigrostriatal dopamine terminal imaging with dopamine transporter SPECT: an update. J Nucl Med 54:1331–1338
Van Laere K, Everaert L, Annemans L, Gonce M, Vandenberghe W, Vander Borght T (2008) The cost effectiveness of 123I-FP-CIT SPECT imaging in patients with an uncertain clinical diagnosis of parkinsonism. Eur J Nucl Med Mol Imaging 35:1367–1376
Walker Z, Costa DC, Walker RW et al (2002) Differentiation of dementia with Lewy bodies from Alzheimer's disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry 73:134–140
Darcourt J, Booij J, Tatsch K et al (2010) EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging 37:443–450
Djang DS, Janssen MJ, Bohnen N et al (2012) SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J Nucl Med 53:154–163
Soderlund TA, Dickson JC, Prvulovich E et al (2013) Value of semiquantitative analysis for clinical reporting of 123I-2-beta-carbomethoxy-3beta-(4-iodophenyl)-N-(3-fluoropropyl)nortropane SPECT studies. J Nucl Med 54:714–722
Tatsch K, Poepperl G (2012) Quantitative approaches to dopaminergic brain imaging. Q J Nucl Med Mol Imaging 56:27–38
Zubal IG, Early M, Yuan O, Jennings D, Marek K, Seibyl JP (2007) Optimized, automated striatal uptake analysis applied to SPECT brain scans of Parkinson's disease patients. J Nucl Med 48:857–864
Soret M, Koulibaly PM, Darcourt J, Hapdey S, Buvat I (2003) Quantitative accuracy of dopaminergic neurotransmission imaging with (123)I SPECT. J Nucl Med 44:1184–1193
Chang LT (1978) Method for attenuation correction in radionuclide computed tomography. Ieee Transactions on Nuclear Science 25:638–643
Zaidi H, Hasegawa B (2003) Determination of the attenuation map in emission tomography. J Nucl Med 44:291–315
Hosoba M, Wani H, Toyama H, Murata H, Tanaka E (1986) Automated body contour detection in SPECT: effects on quantitative studies. J Nucl Med 27:1184–1191
Larsson SA (1980) Gamma camera emission tomography. Development and properties of a multi-sectional emission computed tomography system. Acta Radiol Suppl 363:1–75
Macey DJ, DeNardo GL, DeNardo SJ (1988) Comparison of three boundary detection methods for SPECT using Compton scattered photons. J Nucl Med 29:203–207
Lange C, Seese A, Schwarzenbock S et al (2014) CT-based attenuation correction in I-123-ioflupane SPECT. PLoS One 9:e108328
Hulme KW, Kappadath SC (2014) Implications of CT noise and artifacts for quantitative 99mTc SPECT/CT imaging. Med Phys 41:042502
Koch W, Radau PE, Hamann C, Tatsch K (2005) Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med 46:1109–1118
Frackowiak RSJ, Friston KJ, Frith CD et al (2004) Human brain function. Academic Press, San Diego
Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C (1998) A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inform (Lond) 23:207–214
Dickson JC, Tossici-Bolt L, Sera T et al (2010) The impact of reconstruction method on the quantification of DaTSCAN images. Eur J Nucl Med Mol Imaging 37:23–35
Koch W, Hamann C, Welsch J, Popperl G, Radau PE, Tatsch K (2005) Is iterative reconstruction an alternative to filtered backprojection in routine processing of dopamine transporter SPECT studies? J Nucl Med 46:1804–1811
Winz OH, Hellwig S, Mix M et al (2012) Image quality and data quantification in dopamine transporter SPECT: advantage of 3-dimensional OSEM reconstruction? Clin Nucl Med 37:866–871
Ichihara T, Ogawa K, Motomura N, Kubo A, Hashimoto S (1993) Compton scatter compensation using the triple-energy window method for single-isotope and dual-isotope spect. J Nucl Med 34:2216–2221
Buchert R, Berding G, Wilke F et al (2006) IBZM tool: a fully automated expert system for the evaluation of IBZM SPECT studies. Eur J Nucl Med Mol Imaging 33:1073–1083
Innis RB, Cunningham VJ, Delforge J et al (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539
Benamer HTS, Patterson J, Grosset DG et al (2000) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [I-123]-FP-CIT SPECT imaging: The [I-123]-FP-CIT study group. Mov Disord 15:503–510
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Booij J, Habraken JB, Bergmans P et al (1998) Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson's disease. J Nucl Med 39:1879–1884
Tsuchida T, Ballinger JR, Vines D et al (2004) Reproducibility of dopamine transporter density measured with 123I-FPCIT SPECT in normal control and Parkinson's disease patients. Ann Nucl Med 18:609–616
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Oh M, Kim JS, Kim JY et al (2012) Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med 53:399–406
Scherfler C, Schwarz J, Antonini A et al (2007) Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov Disord 22:1229–1238
Schwarz J, Storch A, Koch W, Pogarell O, Radau PE, Tatsch K (2004) Loss of dopamine transporter binding in Parkinson's disease follows a single exponential rather than linear decline. J Nucl Med 45:1694–1697
Acknowledgments
The scientific guarantor of this publication is Ralph Buchert. The authors of this manuscript declare relationships with the following companies: Bert Umland-Seidler is an employee of GE Healthcare Buchler GmbH & Co. KG. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Study subjects were previously reported in Lange C, Seese A, Schwarzenbock S, et al. (2014) CT-Based Attenuation Correction in I-123-Ioflupane SPECT. PLoS One 9:e108328. Methodology: retrospective, diagnostic study, multicenter study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lange, C., Kurth, J., Seese, A. et al. Robust, fully automatic delineation of the head contour by stereotactical normalization for attenuation correction according to Chang in dopamine transporter scintigraphy. Eur Radiol 25, 2709–2717 (2015). https://doi.org/10.1007/s00330-015-3667-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3667-6