Abstract
Purpose
Our primary endpoint was to assess pathological response rate (pT0N0 and ≤pT1N0) for patients with BCa treated with the accelerated or dose dense MVAC (ddMVAC) chemotherapy followed by radical cystectomy (RC) in this real-word multi-institutional cohort.
Materials and methods
We retrospectively reviewed records of patients with urothelial cancer who underwent ddMVAC and RC at seven contributing institutions from 2000 to 2015. Patients with cT2–4a, M0 BCa were included. Presence of cT3–4 disease, hydronephrosis, lymphovascular invasion and/or existence of sarcomatoid, or micropapillary features on the initial transurethral resection of bladder tumor specimen was defined as high-risk disease. Logistic regression models for prediction of pT0N0 and ≤pT1N0 were generated for the entire cohort as well as for the cN0 subgroup. The multivariable Cox proportional hazards regression model for survival using post RC data was used to assess hazard ratios (HRs) for the variables of interest.
Results
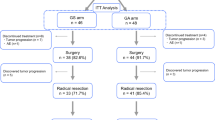
A total of 345 patients received ddMVAC chemotherapy during the study period; 85% had high-risk features. The median number of chemotherapy cycles was 4 (IQR 4-4); >90% of patients completed all scheduled cycles. The observed rates of pT0N0 and ≤pT1N0 were 30.4 and 49.3%, respectively, among cN0 patients. On the multivariable regression model, the presence of more than one clinical high-risk element was associated with 70% [OR 0.30 95% CI (0.10–0.86); p = 0.02] reduction in the odds of achieving partial pathological response.
Conclusions
A complete response (pT0N0) was observed in one-third of patients after neoadjuvant ddMVAC therapy, and a partial response (≤pT1N0) was observed in nearly half of the cases in this real-world experience with this regimen. To our knowledge, this represents the largest experience outside clinical trial settings.

Similar content being viewed by others
References
Grossman HB, Natale RB, Tangen CM et al (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349(9):859–866
Advanced Bladder Cancer (ABC) Meta-analysis Collaboration (2005) Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 48(2):202–205
Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK (2011) International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 29(16):2171–2177
Zargar-Shoshtari K, Zargar H, Lotan Y et al (2016) A multi-institutional analysis of outcomes of patients with clinically node positive urothelial bladder cancer treated with induction chemotherapy and radical cystectomy. J Urol 195(1):53–59
Meijer RP, Nieuwenhuijzen JA, Meinhardt W et al (2013) Response to induction chemotherapy and surgery in non-organ confined bladder cancer: a single institution experience. Eur J Surg Oncol 39(4):365–371
Zaid HB, Patel SG, Stimson CJ et al (2014) Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 83(1):75–80
Gray PJ, Fedewa SA, Shipley WU et al (2013) Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol 63(5):823–829
Kassouf W (2014) Uptake of neoadjuvant chemotherapy for invasive bladder cancer. Can Urol Assoc J 8(3–4):e294–e295
Zargar H, Espiritu PN, Fairey AS et al (2015) Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 67(2):241–249
von der Maase H, Sengelov L, Roberts JT et al (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23(21):4602–4608
Sternberg CN, de Mulder PH, Schornagel JH et al (2001) Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 19(10):2638–2646
Blick C, Hall P, Pwint T et al (2012) Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (AMVAC) as neoadjuvant chemotherapy for patients with muscle-invasive transitional cell carcinoma of the bladder. Cancer 118(16):3920–3927
Plimack ER, Hoffman-Censits JH, Viterbo R et al (2014) Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 32(18):1895–1901
Choueiri TK, Jacobus S, Bellmunt J et al (2014) Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 32(18):1889–1894
Culp SH, Dickstein RJ, Grossman HB et al (2014) Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol 191(1):40–47
Millikan R, Dinney C, Swanson D et al (2001) Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol 19(20):4005–4013
Mead GM, Russell M, Clark P et al (1998) A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: results and a report on prognostic factors in a Medical Research Council study. MRC Advanced Bladder Cancer Working Party. Br J Cancer 78(8):1067–1075
Galsky MD, Pal SK, Chowdhury S et al (2015) Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer 121(15):2586–2593
Sonpavde G, Goldman BH, Speights VO et al (2009) Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 115(18):4104–4109
Zargar H, Zargar-Shoshtari K, Lotan Y et al (2015) Final pathological stage after neoadjuvant chemotherapy and radical cystectomy for bladder cancer-does pT0 predict better survival than pTa/Tis/T1? J Urol 195:886–893
Christodouleas JP, Baumann BC, He J et al (2014) Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 8710. Cancer 120(8):1272–1280
Zargar H, Zargar-Shoshtari K, Dundee P, Black PC (2016) Predicting occult lymph node positive disease at the time of radical cystectomy: a systematic review. Minerva Urol Nefrol 68(2):112–124
Monn MF, Kaimakliotis HZ, Pedrosa JA et al (2015) Contemporary bladder cancer: variant histology may be a significant driver of disease. Urol Oncol 33(1):18
Meeks JJ, Taylor JM, Matsushita K et al (2013) Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int 111(8):E325–E330
Ghoneim IA, Miocinovic R, Stephenson AJ et al (2011) Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology 77(4):867–870
Cancer Genome Atlas Research N (2014) Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507(7492):315–322
Choi W, Porten S, Kim S et al (2014) Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25(2):152–165
McConkey DJ, Choi W, Shen Y et al (2015) A prognostic gene expression signature in the molecular classification of chemotherapy-naive urothelial cancer is predictive of clinical outcomes from neoadjuvant chemotherapy: a phase 2 trial of dose-dense methotrexate, doxorubicin, vinblastine, and cisplatin with bevacizumab in urothelial cancer. Eur Urol. doi:10.1016/j.eururo.2015.08.034
Van Allen EM, Mouw KW, Kim P et al (2014) Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 4(10):1140–1153
Groenendijk FH, de Jong J, Fransen van de Putte EE et al (2016) ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol 69(3):384–388
Plimack ER, Dunbrack RL, Brennan TA et al (2015) Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 68(6):959–967
Acknowledgements
The authors would like to acknowledge James Vanhie for his contribution toward data collection.
Author information
Authors and Affiliations
Contributions
Author contributions
The authors listed below have made substantial contributions to the intellectual content of the paper described below. Homayoun Zargar: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing; Jay B. Shah: Data analysis, Manuscript writing/editing; Elisabeth E. Fransen van de Putte: Protocol/project development, Data collection or management; Kylea R. Potvin: Protocol/project development, Data collection or management; Kamran Zargar-Shoshtari: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing; Bas W. van Rhijn: Data analysis, Manuscript writing/editing; Siamak Daneshmand: Data analysis, Manuscript writing/editing; Jeff M. Holzbeierlein: Data analysis, Manuscript writing/editing; Philippe E. Spiess: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing; Eric Winquist: Data analysis, Manuscript writing/editing; Simon Horenblas: Data analysis, Manuscript writing/editing; Colin Dinney: Data analysis, Manuscript writing/editing; Peter C. Black: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing; Wassim Kassouf: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict if interest relation to this work.
Ethical approval
Ethical standards have been observed during conduction and reporting of this work. Institutional review Board has approved the protocol for this study.
Rights and permissions
About this article
Cite this article
Zargar, H., Shah, J.B., van de Putte, E.E.F. et al. Dose dense MVAC prior to radical cystectomy: a real-world experience. World J Urol 35, 1729–1736 (2017). https://doi.org/10.1007/s00345-017-2065-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-017-2065-x