Abstract
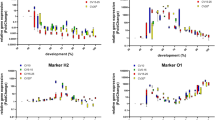
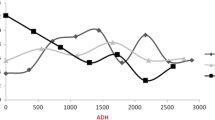
Necrophagous blow fly larvae can provide accurate estimates of the minimum postmortem interval in death investigations. During larval development, predictable morphological changes occur and measurements of weight, length, and width are compared to species-specific growth curves for reliable age estimates. However, aging blow fly pupae is more challenging because morphological and anatomical changes are not visible with the naked eye. Thus, delicate preparation of the pupae or rearing to the adult stage seems unavoidable. Conversely, metamorphosis evokes a remodelling of the larval shape to adult structures, and gene expression analysis potentially serves as a molecular tool to mirror the ageing process of a pupa. The present study focuses on the differential expression of two newly described, arbitrarily named genes (15_2, 2014192) and two previously identified genes (actin, arylphorin receptor) during Calliphora vicina (Diptera: Calliphoridae) metamorphosis. Quantification through real-time PCR revealed significant up- and downregulation of these transcripts found to be temperature dependent and age specific, hence, a new possibility to age forensically important blow fly pupae.


Similar content being viewed by others
References
Smith KGV (1986) A manual of forensic entomology. Cornell University Press, London
Villet MH, Amendt J (2011) Advances in entomological methods for death time estimation. In: Turk EE (ed) Forensic Pathology Reviews doi:10.1007/978-1-61779-249-6_11.
Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MJR (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol doi:10.1007/s12024-010-9209-2
Forbes SI, Dadour I (2010) The soil environment and forensic entomology. In: Byrd JH and Castner JL (eds) Forensic entomology—The utility of arthropods in legal investigations, 2nd edn. CRC, Boca Raton, p 416
Richards CS, Crous KL, Villet M (2009) Models of development for blowfly sister species Chrysomya chloropyga and Chrysomya putoria. Med Vet Entomol 23:56–61
Grassberger M, Reiter C (2001) Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen- and isomorphen-diagram. Forensic Sci Int 120:32–36
Wells JD, LaMotte LR (1995) Estimating maggot age from weight using inverse prediction. J Forensic Sci 40:585–590
Greenberg B (1991) Flies as forensic indicators. J Med Entomol 28:565–577
Reiter C (1984) Zum Wachstumsverhalten der Maden der blauen Schmeißfliege Calliphora vicina. Zeitschrift für Rechtsmedizin, 91:295–308
Marchenko MI (2001) Medicolegal relevance of cadaver entomofauna for the determination of the time of death. Forensic Sci Int 120:89–109
Zajac BK (2011) Morphologische und histologische Methoden zur Bestimmung des Alters forensisch relevanter Fliegenpuppen. Diplomarbeit. Goethe-Universität Frankfurt am Main
Bainbridge SP, Bownes M (1981) Staging the metamorphosis of Drosophila melangaster. J Embryol exp Morph 66:57–80
Robertson CW (1936) The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J Morphol 59:351–399.
Beckstead RB, Lam G, Thummel CS (2005) The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol 6:R99
Thummel CS (1996) Flies on steroids—Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet 12:306–310
Buszczak M, Segraves WA (2000) Insect metamorphosis: out with the old, in with the new. Curr Biol 10:R830–R833
White KP, Rifkin SA, Hurban P, Hogness DS (1999) Microarray analysis of Drosophila development during metamorphosis. Science 286:2179–2184
Bowen ID, Mullarkey K, Morgan SM (1996) Programmed cell death during metamorphosis in the blow-fly Calliphora vomitoria. Microsc Res Tech 34:202–217
Tarone AM, Jennings KC, Foran DR (2007) Aging blow fly eggs using gene expression: a feasibility study. J Forensic Sci 52:1350–1354
Tarone AM, Foran DR (2011) Gene expression during blow fly development: improving the precision of age estimates in forensic entomology. J Forensic Sci 56:S112–S122.
Ames C, Turner B, Daniel B (2006) Estimating the post-mortem interval (II): the use of differential temporal gene expression to determine the age of blowfly pupae. International Congress Series 1288:861–863
Gaudry E, Blais C, Annick M, Dauphin-Villemant C (2006) Study of steroidogenesis in pupae of the forensically important blow fly Protophormia terraenovae (Robineau-Desvoidy) (Diptera: Calliphoridae). Forensic Sci Int 160:27–34
Mösch SA (2005) Molekularbiologische Altersbestimmung an Puppen der forensisch relevanten Schmeißfliege Lucilia sericata. Diplomarbeit, Fachhochschule Aachen.
Kim YJ, Kwak CI, Gu YY, Hwang IT, Chun JY (2004) Annealing control primer system for identification of differentially expressed genes on agarose gels. BioTech 36:424–434
Hwang IT, Kim YJ, Kim SH, Kwak CI, Gu YY, Chun JY (2003) Annealing control primer system for improving specificity of PCR amplification. BioTech 35:2–6
Rognes K (1991) Blowflies (Diptera, Calliphoridae) of Fennoscandia and Denmark. E.J. Brill/Scandinavian Science, Leiden
GeneFishing™ DEG Premix Kit, User Manual, Seegene
Beckmann B (2004) Studien der Genexpressionsänderung während der Entwicklung von Drosophila melanogaster mittels DNA-Microarrays. Dissertation. Ruprecht-Karls-Universität Heidelberg
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta (CT)) method. Methods 25:402–408
Pfaffl MW (2001) A new mathematical model for relative quantificationin real-time RT-PCR. Nucleic Acids Res 29: e45
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: research0034.1-research0034.11
Ramakers C, Ruijter JM, Lekanne Deprez RH, Moorman AFM (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339:62–66
Burmester T, Scheller K (1997) Developmentally controlled cleavage of the Calliphora arylphorin receptor and posttranslational action of the steroid hormone 20-hydroxyecdysone. Eur J Biochem 247:695–702
Tarone AM, Picard CJ, Spiegelman C, Foran DR (2011) Population and temperature effects on Lucilia sericata (Diptera: Calliphoridae) body size and minimum developmental time. J Med Entomol 48:1062–1068.
Gallagher MB, Sandhu S, Kimsey R (2010) Variation in developmental time for geographically distinct populations of the common green bottle fly, Lucilia sericata (Meigen). J Forensic Sci 55:438–442
Acknowledgments
This project was financially supported by the Deutsche Forschungsgemeinschaft (project number: ZE 501/2-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boehme, P., Spahn, P., Amendt, J. et al. Differential gene expression during metamorphosis: a promising approach for age estimation of forensically important Calliphora vicina pupae (Diptera: Calliphoridae). Int J Legal Med 127, 243–249 (2013). https://doi.org/10.1007/s00414-012-0699-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-012-0699-1