Abstract
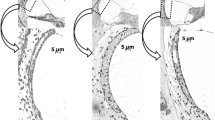
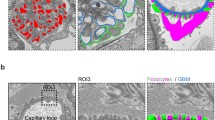
The loss of the function of the peroxisomal Mpv17-protein and associated imbalanced radical oxygen species (ROS) homeostasis leads to an early onset of focal segmental glomerulosclerosis and sensorineural deafness associated with severe degeneration of cochlear structures. An excessive enlargement of basal laminae of the stria vascularis capillaries and glomeruli indicates numerous changes in their molecular composition. The basement membrane (BM) of the glomeruli and the stria vascularis are simultaneously affected in early stages of the disease and the lamination, splitting of the membrane and formation of the “basket weaving” seen at the onset of the disease in the kidney are similar to the ultrastructural alterations characteristic for Alportȁ9s syndrome. The progressive alteration of the BMs is accompanied by irregularity in the distribution of the collagen IV subunits and by an accumulation of the laminin B2(γ1) in the inner ear and B(β1) in the kidney. Since Mpv17 protein contributes to ROS homeostasis, further studies are necessary to elucidate downstream signaling molecules activated by ROS. These studies explain the cellular responses to missing Mpv17-protein, such as accumulation of the extracellular matrix, degeneration, and apoptosis in the inner ear.




Similar content being viewed by others
References
Abrahamson DR, Prettyman AC, Robert B, St John PL (2003) Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int 63:826–834
Binder CJ, Weiher H, Exner M, Kerjaschki D (1999) Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot processes flattening ad proteinuria. J Pathol 154:1067–1075
Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Santes J, Timpl R, Trygggvason K, Yamada Y, Yurchenko PD (1994) A new nomenclature for the laminin. Matrix Biol 14:209–211
Chai Q, Krag S, Miner JH, Neyengaard JR, Chai S, Wogensen L (2003) TGF-β1 induces aberrant laminin chain and collagen type IV isotype expression in the glomerular basement membrane. Nephron Exp Nephrol 94:123–136
Cosgrove D, Rodgers KD (1997) Expression of the major basement membrane-associated proteins during postnatal development in the murine cochlea. Hear Res 105:159–170
Cosgrove D, Samuelson G, Pitt J (1996a) Immunohistochemical localization of basement membrane collagens and associated proteins in the murine cochlea. Hear Res 97:54–65
Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC (1996b) Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev 10:2981–2992
Cosgrove D, Samuelson GC, Meehan DT, Miller C, McGee J, Walsh EJ, Siegel M (1998) Ultrastructural, physiological and molecular defects in the inner ear of a gene-knockout mouse model for autosomal Alport syndrome. Hear Res 121:84–98
Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R (2000) Integrin alpha1 beta1 and transforming growth factor-beta1 play distinct role in Alport glomerular pathogenesis and serve as dual target for metabolic therapy. Am J Pathol 157:1649–1659
Erickson AC, Couchman JR (2000) Still more complexity in mammalian basement membrane. J Histochem Cytochem 48:1291–1306
Fassin G, Ferrari N, Brigati C Benelli R, Santi L, Noonan DM, Albini A (2000) Tissue inhibitors of metaloproteinases: regulation and biological activities. Clin Exp Metastasis 18:111–120
Gibson IW, More IAR (1998) Glomerular pathology: recent advances. J Pathol 184:123–129
Gratton MA, Schulte BA (1995) Alterations in microvasculature are associated with atrophy of stria vascularis in quiet-aged gerbils. Hear Res 82:44–52
Gratton MA, Schmied RA, Schulte BA (1996) Age-related decreases in endocochlear potential are associated with vascular abnormalities. Hear Res 102:181–190
Gratton MA, Meekan DT, Smyth BJ, Cosgrove D (2002.) Strial marginal cells play a role in basement membrane homeostasis: in vitro and in vivo evidence. Hear Res 163:27–36
Gross O, Beirowski B, Koepke ML, Kuck J, Reiner M, Addick K, Smyth N, Schulze-Lohoff, Weber M (2003) Preemptive ramipril therapy delay renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int 63:438–446
Ha H, Lee HB (2001) Oxidative stress in diabetic nephropathy: basic and clinical information. Curr Diab Rep 1:282–287
Iida R, Yasuda T, Tsubota E, Matsuki T, KishiK (2001) Cloning, mapping, genomic organization, and expression of mouse M-LP, a new member of peroxisomal membrane protein Mpv17 domain family. Biochem Biophys Res Commun 283:292–296
Iida R, Yasuda T, Tsubota E, Takatsuka H, Masuyama M, Matsuki T, Kishi K (2002) M-LP. Mpv17-like protein, has a peroxisomal membrane targeting signal comprising a transmembrane domain and a positively charged loop and upregulates expression of the manganese superoxide dismutase gene. J Biol Chem 278:6301–6306
Iida R, Yasuda T, Tsubota E, Takatsuka H, Masuyama M, Matsuki T, Kishi K (2005) A novel alternative spliced Mpv17-like protein isoform localizes in cytosol and is expressed in a kidney- and adult-specific manner. Exp Cell Res 302:22–30
Izzedine H, Tankere F, Launay-Vacher W, Deray G (2004) Ear and kidney syndromes: molecular vs clinical approach. Kidney Int 65:369–385
Kaldi K, Diestelkotter P, Stenbeck G, Aurbach S, Jakle U, Magert HJ, Wieland FT, Just WW (1993) Membrane topology of the 22 kDa integral peroxisomal membrane protein. FEBS Lett 315:217–222
Kashtan CE (1999) Alport syndrome, an inherited disorder of renal, ocular, and cochlear basement membrane. Medicine 78:338–360
Kashtan CE, Kim Y, Lees GE, Thorner PS, Vitrtanen I, Miner JH (2000) Abnormal glomerular basement membrane laminins in murine, canine and human Alport syndrome: aberrant laminin α2 deposition is species independent. J Am Soc Nephrol 12:252–260
Mason RM, Wahab NA (2003) Extracellular matrix metabolism in diabetic nephropathy. J Nephrol 14:1358–1373
McGabe BF (1979) Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol 88:585–589
McQueen CT, Baxter A, Smith TL, Raynor R, Yoon SM, Prazma J, Pillsbury HC (1999) Non insulin dependent diabetic microangiopathy in the inner ear. J Laryngol Otol 113:13–18
Merchant SN, Burgess BJ, Adams JC, Kashtan CE; Gregory MC, Santi PA, Colvin R, Collins B, Nadol JB (2004) Temporal bone histopathology in Alport syndrme. Laryngoscope 114:1609–1618
Meyer zum Gottesbeger AM, Felix H, Reuter A, Weieher H (2001a) Ultrastructural and physiological effects in the cochlea of the Mpv17 mouse strain. A comparison between young and old adult animals. Hear Res 156:69–80
Meyer zum Gottesberge AM, Reuter A, Weiher H (1996) Inner ear defect similar to Alportȁ9s syndrome in the glomerulosclerosis mouse model Mpv17. Eur Arch Otorhinolaryngol 253:470–474
Meyer zum Gottesberge AM, Balz V, Felix H (2001b) Mpv17-/- mouse strain a model for the relationship between the kidney and the inner ear. Adv Otorhinolaryngol 59:84–90
Miner JH (2003) A molecular look at the glomerular barrier. Nephron Exp Nephrol 94:119–122
Miner JH, Sanes JS (1996) Molecular and functional defects in kidneys of mice lacking collagen α3(IV): implications for Alport syndrome. J Cell Biol 135:1403–1413
Miner JH, Yurchenco PD (2004) Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 20:155–284
Müller M, Smolders JWT, Meyer zum Gottesberge AM, Reuter A, Zwacka RM, Weiher H, Klinke R (1997) Loss of auditory function in transgenic Mpv17-deficient mice. Hear Res 114:259–263
Noakes PG, Minner JH, Gautam M, Cunningham JM, Santes JR, Merlie JP (1995) The renal glomerulus of mice lacking s-laminin/laminin beta2: nephrosis despite molecular compensation by laminin beta1. Nat Genet 10:400–406
Oȁ9Bryan T, Weiher H, Rennke HG, Kren S, Hostetter TH (2000) Course of renal injury in the Mpv17-deficient transgenic mouse. J Am Soc Nephrol 11:1067–1074
Peters TA, Monnens LAH, Cremers CWRJ, Curfs JHAJ (2004) Genetic disorders of transporters/channels in the inner ear and their relation to the kidney. Pediatr Nephrol 19:1194–1201
Rao VH, Lees GE, Kashtan CE, Nemori R, Singh RK, Meehan DT, Rodgers K, Berridge BR, Bhattacharya G, Cosgrove D (2003) Increased expression of MMp-2, MMP-9 (type IV collagenase/gelatinase), and MTI– mMP in canine X-linked Alport syndrome (XLAS). Kidney Int 63:1736–1748
Reuter A, Nestl A, Zwacka RM, Tuckermann J, Waldherr R, Wagner EM, Höyhtyä, M, Meyer zum Gottesberge AM, Angel P, Weiher H (1998) Expression of the recessive glomerulosclerosis gene Mpv17 regulates MMP-2 expression in fibroblasts, the kidney and the inner ear. Mol Biol Cell 9:1675–1682
Rodgers KD, Barrit L, Miner JH, Cosgrove D (2001) The laminins in the murine inner ear: developmental transitions and expression in cochlear basement membranes. Hear Res 158:39–50
Rohrmoser MM, Mayer G (1996) Reactive oxygen species and glomerular injury. Kidney Blood Press Res 19:263–269
Sakagutchi N, Spicer SS, Thomopoulos GN, Schulte BA (1997) Immunoglobulin deposition in thickened basement membranes of aging strial capillaries. Hear Res 109:83–91
Sayers R, Kalluri R, Rodgers KD, Shield CF, Meeehan T, Cosgrove D (2001) Role for transforming growth factor-β1 in Alport renal disease progression. Kidney Int 56:1662–1673
Schucknecht HF (1974) Developmental defects. In: Pathology of the ear. Harvard University Press, Cambridge, MA, pp165–213
Slepecky N (1996) Structure of the mammalian cochlea. In: Dallos P, Popper AN, Fay RR (eds) The cochlea. Springer, Berlin Heidelberg New York, pp 44–129
Smith TL, Raynir E, Prazma J, Buenting JE, Piilsbury HC III (1995) Insulin dependent diabetic microangiopathy in the inner ear. Laryngoscope 105:236–240
Strauß P, Schmittner S, Rick W, Fassbender-Balg S (1982) Inner ear and diabetes mellitus diabetic mutant mice. Laryngol Rhinol Otol 61:325–330
Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA (1997) Age-related thickening of basement membrane in stria vascularis capillaries. Hear Res 111:31–41
Timpl R, Brown JC (1996) The laminins. Matrix Biol 14:275–281
Tokahashi M, Hokunan K (1992) Localization of type IV collagen and laminin in the guinea pig inner ear. Ann Otol Rhinol Laryngol 191:58–62
Tsupran V, Santi P (1999) Ultrastructure and immunohistochemical identification of the extracellular matrix of the chinchilla cochlea. Hear Res 129:35–49
Tsupran V, Santi P (2001) Proteoglycan arrays in the cochlear basement membrane. Hear Res 175:65–76
Tunggal P, Smyth N, Paulsson M, Ott MC (2000) Laminins: structure and genetic regulation. Microsc Res Tech 51:214–227
Wagner G, Stettmaier K, Bors W, Sies H, Wagner EM, Reuter A, Weiher H (2001) Enhanced gamma-glutamyl transpeptidase expression and superoxide production in Mpv17-/-glomerulosclerosis mice. Biol Chem 382:1019–1025
Weidauer H, Arnold A (1976) Structurelle Veränderungen am Hörorgan beim Alport Syndrom. Z Laryngol Rhinol Otol 55:6–16
Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R (1990) Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell 62:425–434
Weinberger DG, ten-Cate WJ, Lautermann J, Baethmann M (1999) Lacalization of laminin isoforms in the guinea pig cochlea. Laryngoscope 109:201–204
Yoshizaki T, Sato H, Furukawa M (2002) Recent advances in the regulation of matrix metalloproteinas 2 activation: from basic research to clinical implication (review). Oncol Rep 9:607–611
Yurchenko PD, Schittny JS (1990) Molecular architecture of basement membranes. FASEB J 4:1577–1590
Zwacka R, Reuter A, Pfaff E, Moll J, Gorgas K, Karasawa M, Weiher H (1994) The glomerulosclerosis gene Mpv17 encodes a peroxisomal protein producing reactive oxygen species. EMBO J 13:5129–5134
Acknowledgments
The authors wish to thank Mrs. V. Hoffmann (Zürich) and U. Becker-Lendzian for their excellent technical help, Mrs.Trenker for the care of the animal and Dr. M.A. Gratton (University of Pennsylvania) for critical comments to the manuscript. Also we would like to acknowledge Dr. J. Miner Washington University for providing rabbit anti-novel chains collagen 4A3, 4A4, and 4A5 antibodies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meyer zum Gottesberge, A.M., Felix, H. Abnormal basement membrane in the inner ear and the kidney of the Mpv17-/- mouse strain: ultrastructural and immunohistochemical investigations. Histochem Cell Biol 124, 507–516 (2005). https://doi.org/10.1007/s00418-005-0027-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0027-7