Abstract
Activation of CD8+ cytotoxic T cells is crucial for the adaptive immune response against viral infections and the control of malignant transformed cells. Together with activation of costimulatory molecules like CD3 and CD28, CD8+ T cells need activation of their unique T cell receptor via recognition of foreign peptide epitopes in combination with major histocompatibility complexes class I on the cell surface of professional antigen-presenting cells. Presentation of pathogen-associated proteins is the result of a complex proteolytic process. It starts with the breakdown of proteins by a cytosolic endopeptidase, the proteasome, and is continued by subsequent N-terminal trimming events in the cytosol and/or the endoplasmic reticulum. Analysis of the proteolytic aminopeptidase activity in the former cellular compartment showed that the cytosol harbors a multitude of aminopeptidases that have singular specificities, but on the other hand also show redundancy in the trimming of N-terminal residues. The observed pattern of the overall trimming in the cytosol is reflected by the activity of the four identified aminopeptidases, and the administration of protease inhibitors made it possible to assign specificity of cleaving of proteinogenic amino acids to one or more identified aminopeptidase. The only exception was the cleavage of aspartic acid, which is performed by one yet unidentified enzyme.
Similar content being viewed by others
Introduction
Generation of major histocompatibility complex I (MHC I)–restricted peptide epitopes is the result of a complex proteolytic process requiring different steps performed either in the cytosol or in the endoplasmic reticulum (ER). Activation of cytotoxic T-lymphocytes (CTLs) for the defense against viral or bacterial infections is strongly dependent on proper processing of intracellular pathogen-derived proteins and subsequent presentation of peptide epitopes bound to MHC I molecules on the cell surface of professional antigen-presenting cells (reviewed in [1, 2]). The first step of peptide generation is dependent on the proteolytic activity of a large cytosolic endopeptidase complex, the proteasome [3]. Proteasomal cleavage of substrate proteins generates peptides with the length of 5–25 amino acids, which in most cases already possess the correct C-terminus of the mature epitope. Regarding the length of MHC I-restricted epitopes, normally ranging from 8 to 11 amino acids, the proteasome generates mature epitopes or N-terminally extended epitope precursors, as well as generating peptides too short for presentation [4, 5]. In general, the cytosol is a very aggressive environment for proteins and peptides, and it is estimated that peptides that injected in the cytosolic compartment have a half-life of only 5 s [6]. This is due to the vast presence of additional proteases and peptidases in this compartment besides the proteasome, some of which already have been implicated in the antigen-processing pathway for MHC I molecules [7, 8]. The puromycin-sensitive aminopeptidase (PSA), for example, is a cytosolic member of the M1 family of metallopeptidases. It has been shown that knockout of the enzyme enhances MHC class I surface expression as PSA diminishes the supply of suitable peptides available for loading onto MHC I molecules if the peptides are not rescued by the action of the transporter associated with antigen processing (TAP) [9]. On the other hand, knockout of another cytosolic peptidase, the Bleomycin Hydrolase (BH) does not change MHC class I expression [10]. In the ER, the aminopeptidases (APs) ERAP1 and ERAP2 trim N-terminally extended epitope precursors to fit the requirements for binding on MHC I molecules [11]. Taken together, both cytosolic as well as ER-resident APs have an important impact on MHC class I antigen processing [12]. Here, we quantitatively determine the different AP activities in the cytosolic compartment. Identification and characterization of involved APs deepens our knowledge of the antigen presentation pathway and therefore provides us the opportunity to manipulate proteolytic steps via specific protease inhibitors to influence antigen presentation and activation of CTLs in both disease and vaccination.
Materials and methods
Cell lines and mouse strains
Cells lines (HeLa, MC57, RMA, J774, EL4, D2SC/1, RAW309) were grown in RPMI 1640 (Biowhittaker) or ISCOVE’s (10 % FCS; 2 mM glutamine; 1 mM sodium pyruvate; 100 μ/ml Penicillin/100 μg/ml Streptomycin) and were incubated at 37 °C, 5 % CO2. Used mouse strains were Blmh−/− on C57BL/6 background [13], Goku on BALB/c background [14], BALB/c and C57BL/6 (Charles River Laboratories).
Recombinant enzymes
Human Bleomycin Hydrolase was expressed recombinantly in E. coli and was a kind gift of J.S. Lazo, Pittsburgh, USA.
Puromycin-sensitive aminopeptidase was expressed in baculovirus-infected Sf9 cells and kindly provided from M.W. Thompson, working group L.B. Hersh, USA.
Compartment separation
Cytosolic purification was based on the protocol of Walter and Blobel [15]. Up to two liters, cell culture was cultivated and adherent cells were detached with 0.3 mM EDTA in PBS; pH 7.2. After harvesting the cells (1,000×g, 5 min, 4 °C), the pellet was washed once with PBS and twice with protease assay (PA) buffer (20 mM HEPES/KOH; pH 7.6; 150 mM KCl; 5 mM MgCl2; 0.5 mM DTT). All following steps were conducted at 4 °C or on ice. Cells were resuspended in 5 ml PA buffer and were homogenized with an Elvehjem potter. Debris, nuclei and mitochondria were removed by centrifugation (1,000×g, 10 min and 10,000×g, 10 min, respectively). The cleared cell lysate was bedded on a sucrose layer (1.3 M sucrose in PA buffer; ratio lysate/sucrose layer 3:1), and cytosol was separated by centrifugation for 2.5 h at 140,000×g. The supernatant, containing the purified cytosol was aliquoted, snap-frozen in liquid nitrogen and stored at −80 °C.
Aminopeptidase activity assay
Aminopeptidase activity was measured in 96-well format in a total volume of 200 μl. For each individual amino acid-AMC (7-amido-4-methylcoumarin) conjugate, wells with 1 mg/ml BSA in PA buffer were prepared and same volume of cytosolic fraction or PA buffer was added. Samples were brought to 37 °C before measurement and in case of inhibitory studies were preincubated for 30 min. Protease inhibitors were used in the following concentrations: puromycin 100 μM, E64 100 μM, arphamenin A 1 μM and Captopril 100 μM. To start the reaction, AMC substrates with single amino acids coupled to the fluorophore 7-Amido-4-methylcoumarin (Bachem) were added in a final concentration of 100 μM, and fluorescence was recorded with an excitation of 360 nm and an emission of 450 nm with a SPECTRAFluor Plus (Tecan). Initial velocity of substrate turnover (v 0) was determined by calculating the slope of relative fluorescence units (RFU) over time (RFU * min−1). These values were normalized to the total volume (RFU * min−1 * μl−1), and to enable the comparison of different data sets, activity for each amino acid was calculated as percentage of total amino acid turnover:
Hydrophobic interaction chromatography
Chromatographic purifications were performed with an ÄKTA purifier system (GE Healthcare) at 4 °C. Cytosol was fractionated via hydrophobic interaction chromatography (HIC). EL-4 cytosolic extract was loaded on a HiTrap Phenyl HP 5-ml column. Buffer A was composed of 50 mM Tris/HCl; pH 7.5; 2 mM MgCl2; 1 mM DTT; 10 mM NaCl; 1 M (NH4)2SO4, and buffer B contained 50 mM Tris/HCl; pH 7.5; 2 mM MgCl2; 1 mM DTT; 10 mM NaCl. A gradient from 0 to 100 % buffer B over 20 column volumes (CV) with a flowrate of 1 ml/min was applied. For the determination of aminopeptidase activity, collected fractions were incubated with methionine (M), proline (P), cysteine (C) and arginine (R) AMC substrate.
Purification of two undefined cytosolic aminopeptidase activities
Aminopeptidases were purified with a multistep strategy of several chromatography techniques described in steps 1–6:
-
1.
HiPrep 16/10 DEAE FF: EL-4 cytosolic extract was diluted with 100-ml ion exchange buffer A (20 mM Tris/HCl; pH 8; 2 mM MgCl2; 1 mM DTT; 10 mM NaCl and loaded on the column. Bound proteins were eluted with a gradient of 0–50 % B (contains buffer A with 500 mM NaCl) in 15 CV and 50–100 % B in 5 CV with a flowrate of 2 ml/min. Collected fractions were tested with indicated AMC substrates. Arginine/proline aminopeptidase eluted in fractions 1F9-1, 1G1-9 (120 mM NaCl), and the cysteine aminopeptidase in fractions 1D11-1, 1E1-7 (80 mM NaCl).
-
2.
(NH4)2SO4 precipitation: in case R/P activity, unwanted proteins were precipitated with 1 M (NH4)2SO4. In case of C activity with 2 M (NH4)2SO4, supernatants were employed for next chromatographic steps.
-
3.
HiTrap Phenyl HP 5 ml: same buffers as in the HIC purification of complete cytosolic extracts were used. R/P supernatant was loaded, and a gradient of 0–40 % B in 4 CV, 40–90 % B in 20 CV and 90–100 % B in 1 CV was applied. C supernatant was eluted with a gradient of 0–50 % B in 5 CV and 50–100 % B in 20 CV. Fractions were tested with R/P or C-AMC substrate, respectively. R/P activity eluted in fractions B4-1, C1-7 (500 mM (NH4)2SO4), and C activity, in fractions B4-1, C1-7 (420 mM (NH4)2SO4).
-
4.
Concentration via spin columns: active fractions were united, and volume was reduced with 10 kDa cutoff Centricon spin columns (Millipore) to 500 μl.
-
5.
Superdex 200 10/300 GL: sample was loaded on the size-exclusion chromatography column, and proteins were separated with a buffer containing 50 mM Tris/HCl; pH 7.5; 2 mM MgCl2; 1 mM DTT; 150 mM NaCl with a flowrate of 0.5 ml/min. R/P activity eluted after 14.25 ml, which corresponds to a size of 74 kDa. C activity eluted after 14.75 ml, which corresponds to a molecular mass of 59 kDa.
-
6.
MiniQ 4.6/50 PE: same buffers as with HiPrep 16/10 DEAE FF were used. Both aminopeptidase were eluted with a gradient from 0 to 60 % B in 15 CV and 60–100 % B in 5 CV with a flowrate of 0.5 ml/min. R/P aminopeptidase eluted in fractions B12-11, and C activity, in fractions A8-10.
SDS Page
Active fractions were concentrated via YM-10 Microcon spin columns (Millipore); sample buffer (1 % SDS, 0.003 % EDTA, 0.01 % bromphenol blue in glycerin) was added, and samples were heated for 5 min to 95 °C. Proteins were separated with a SDS PAGE gel as described earlier. The gel was stained with a zinc staining kit (BioRad), and bands of interest were cut out.
Tryptic digest and mass spectrometric analysis
Gel slices were minced and washed two times with dH20 and 50 % acetonitrile, respectively. Pieces were dried in a SpeedVac concentrator. The pellet was rehydrated in 0.1 M NaHCO3 with 45 mM dithiothreitol and incubated for 1 h at 55 °C. Then, 100 mM 2-iodoacetamide was added and samples were incubated for 1 h at RT. Supernatants were discarded, samples were dried and 0.05 μg/μl Trypsin (Sigma) in 0.1 M NaHCO3 was added to the pellet. Samples were incubated overnight at 37 °C. Extraction of tryptic peptides was performed by incubation with 0.5 % trifluoroacetic acid in dH2O and, subsequently, 0.5 % trifluoroacetic acid in acetonitrile. Pooled supernatants were dried, and peptides were dissolved in 0.1 % trifluoroacetic acid in dH2O. For mass spectrometric analysis, samples were desalted with C18 Ziptips (Millipore) and loaded on the MALDI matrix, containing 2,5-dihydroxy-acetophenone and ammonium citrate in isopropanol (4:1). Tandem mass spectrometric analysis was performed with a MALDI-TOF System (Hewlett-Packard G2025A). M/z ratios and amino acid sequences of detected peptides were evaluated using the Protein Prospector software, University of California, San Francisco (http://prospector.ucsf.edu/). MALDI analysis was done in cooperation with the working group of H.G. Rammensee, Tübingen.
Results
Cytosolic aminopeptidase activity shows distinct preferences toward N-terminal amino acids
After isolation of the cytosolic compartment by differential centrifugation, the cytosolic lysates of a variety of eukaryotic cell lines were screened for AP activity using artificial AP substrates in which single amino acids are coupled to the fluorophore 4-Amino-7-methylcoumarin (AMC). A kinetic was measured, and the activity against all 20 proteinogenic amino acids was determined by calculating the initial slope of emitted fluorescence. As depicted in Fig. 1a, the cytosol displayed a cell type independent pattern of activity, indicating the presence of a set of ubiquitously expressed APs responsible for overall activity in this compartment. Dominating activity was observed for amino acids A, F, K, L, M, P, R and Y.
a Proteolytic activity of cytosolic aminopeptidases in different cell lines. Shown is the metabolic rate for all twenty proteinogenic amino acids as percentage of total turnover. b Specificity of puromycin-sensitive aminopeptidase (PSA) expressed as recombinant enzyme. c Specificity of bleomycin hydrolase (BH) expressed as recombinant enzyme. d Determination of remaining activities in the cytosol after addition of the aminopeptidase inhibitors Puromycin and E64. Amino acids that still being cut with less than 25 % inhibition are marked with asterisks
We decided to identify the proteins responsible for the observed activity pattern. Initially, we analyzed the specificity of two well-characterized APs in the cytosol (namely puromycin-sensitive aminopeptidase, PSA and the Bleomycin Hydrolase, BH). Proteases were recombinantly expressed, and their activity profile was determined. As shown in Fig. 1b, c, PSA and BH clearly differ in their specificities. PSA prefers the amino acids A, F, K, L and M, whereas BH has a broader spectrum but predominantly cuts after C, L, M and Q. Although the activity profile of PSA and BH matches the profile observed in cytosolic extracts, comparison with total cytosolic activity provides evidence for other peptidases involved in total cytosolic amino acid turnover, especially for the amino acids lysine, proline, arginine, but also cysteine.
To further specify the activities of yet unknown APs, cytosolic protease activities were determined in the presence of two AP inhibitors, namely Puromycin (for PSA) and E64 (for BH), and the residual activities were assessed. As depicted in Fig. 1d, especially the activities against C, D, K, P and R are reduced by less than 25 %, suggesting an involvement of other APs in the general turnover of amino acids in the cytosol and responsible especially for those five amino acids.
Hydrophobic interaction HPLC separation of cytosol reveals four distinct peaks of aminopeptidase activity
To characterize cytosolic APs in detail and to determine the enzymes, which could be responsible for the missing activities against C, D, K, P and R, cytosolic cell lysate were separated via hydrophobic interaction chromatography (HIC). After elution of bound proteins, fractions were collected and screened for AP activity against selected AMC substrates, including methionine, a preferred substrate of PSA and BH. As shown in Fig. 2a, the cytosol contains four clearly separated peaks of activity. The first eluting protease in this setting is specific for methionine, whereas the second peak cleaves M, R and C residues. The following peak is strongly active against R but also cuts after P, whereas the last protease is again specific against C. Performing inhibitor studies with Puromycin or E64 (Fig. 2b), peak one and two could be assigned to the two described cytosolic APs: puromycin-sensitive aminopeptidase in fraction C1 and Bleomycin Hydrolase in fraction D1. The two other peaks, however, showed no reduced activity in the presence of both inhibitors. Interestingly, proline-specific protease activity is exclusively found in peak three, whereas cysteine-specific cleavage is performed by BH (peak two) and a so far unknown AP present in peak four. Furthermore, arginine-specific activity derives again from BH (peak 2) but is also detected in peak three. So, besides PSA and BH, the cytosol contains at least two other APs responsible for the activity pattern shown in Fig. 1a.
Identification of two novel cytosolic aminopeptidases
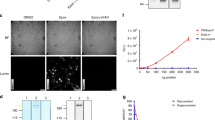
To identify the two unknown APs in peak three and four, cytosolic extract was purified with a multistep strategy of several chromatography techniques (Fig. 3a–g). The cytosol was applied serially to anion exchange, gel-filtration and HIC columns, and after each step, collected fractions were screened for AP activity. Fractions solely active against C or active against P/R were combined and applied onto the next column. Finally, fractions were concentrated and separated by SDS Page (Fig. 3h, i). After Zn-staining, prominent protein bands of interest matching the activity profile were cut out and tryptically digested for MALDI-TOF Tandem-MS analysis. Analysis of m/z values and subsequent databank search of detected protein sequences identified the P/R activity as Aminopeptidase B (AP-B, EC 3.4.11.6) and the P activity as leukotriene A4 hydrolase (LTA4H, EC 3.3.2.6). For both APs, no function in the MHC class I processing pathway has been described so far.
Multistep chromatographic purification of cytosolic aminopeptidases. Left side shows purification of R/P-specific activity, right side shows the purification of C-specific activity. a Aminopeptidase activity of cytosolic extract separated with a HiPrep 16/10 DEAE FF column. b, c: Aminopeptidase activity of HiTrap Phenyl HP eluate fractions. d, e Aminopeptidase activity of Superdex 200 10/300 GL fractions. f, g Activity of MiniQ 4.6/50 PE fractions. h, i Zinc-stained SDS Page
Dissecting cytosolic aminopeptidase activity using specific protease inhibitors
Since the contribution of these proteases to the overall proteolytic activity in the cytosol is unknown, we decided to determine their individual contribution to total cytosolic turnover by using specific protease inhibitors. As a first step, we analyzed the specificity of several known aminopeptidase inhibitors on the four identified proteases in the cytosol (Fig. 4a–d). In detail, we used Puromycin (for PSA), E64 (for BH), Arphamenin (for AP-B) and Captopril (for LTA4H). To avoid unspecific side effects, the inhibitors were titrated using recombinant protein (PSA and BH) or fractions from purified cytosolic extract (AP-B and LTA4H, data not shown). For activity that data shown in Fig. 4, the inhibitors were used in the suitable concentrations (see “Materials and methods”), and activity was determined with L-AMC (PSA and BH, recombinant enzymes), K-AMC (AP-B, purified cytosol) and C-AMC (LTA4H, purified cytosol). Except for Puromycin which inhibited not only PSA completely, but also BH to about 50 %, all tested inhibitors showed to be highly specific in the used concentrations (Fig. 4a–d). E64 was highly specific for BH, not affecting any of the other aminopeptidases tested. Lysine-specific activity of Aminopeptidase B was inhibited for about 80 % by the addition of Arphamenin and Captopril inhibited cysteine activity of LTA4H for about 90 %.
Confirmation of the specificity of used aminopeptidase inhibitors. a–d Aminopeptidase activity with addition of indicated aminopeptidase inhibitors. PSA and BH were administered as recombinant enzymes, and activity was measured against L-AMC. For AP-B and LTA4H, purified cytosol fractions were used and activity was measured against K-AMC (AP-B) or C-AMC (LTA4H), respectively. e, f Splenocyte cell lysates of indicated mouse strains were assayed for aminopeptidase activity with or without Puromycin or E64, and aminopeptidase activity was measured. Shown values were the normalized against P-AMC activity
Since the cytosol may contain additional unknown aminopeptidases, which could be influenced by the administration of inhibitors, the inhibitors Puromycin and E64 were tested in knockout mouse models to exclude unspecific side effects. For this experiment, splenocytes of PSA or BH knockout mice and control mice were lysed, and the lysate was incubated with or without E64 or Puromycin, respectively. Turnover of indicated AMC substrates was determined, and activity was normalized to P activity not affected by PSA or BH. As shown in Fig. 4e, activity of BH is inhibited completely by the addition of E64, reaching values of KO mouse cell lysates which displayed no further inhibition when E64 was added. Therefore, we conclude that no other protease is inhibited, and E64 can be regarded as specific for BH. In the case of Puromycin for which we already have shown that it inhibits not only PSA, but also BH, also the combination of E64 and Puromycin was tested in the PSA knockout model. Disregarding the effect on BH, Puromycin can be considered as highly specific for PSA, and no other AP is affected (Fig. 4f).
PSA, BH, AP-B and LTA4H represent the dominant proteolytic activities in the cytosol
To quantitatively determine the influence of inhibitors on total cytosolic amino acid turnover, EL-4 cytosolic extract was incubated with Puromycin, E64, Arphamenin A or Captopril or a combination of the four, and cleavage of all 20 AMC substrates was determined. Values for each amino acid were calculated as percent of total turnover. Inhibitory patterns now provide information on preferred substrates for each of the four APs detected in the cytosolic extract (Fig. 5).
In case of Puromycin inhibition, it is important to note that besides PSA, also BH is inhibited about 50 %, and also, the values of E64 inhibition should be taken into account. Under these considerations, it becomes evident that PSA is responsible for AP activity directed against the amino acids A, C, F, I, L, M, V, W and Y. BH on the other hand cleaves predominantly E, G, H, N, Q, S and T. As expected, the specificity of AP-B used to isolate the enzyme is reflected in the strong inhibition of cleavage of amino acids K, P and R. To a lesser extent, AP-B removes G, H, N, T, W and Y residues. LTA4H does not seem to play a significant role in overall cytosolic amino acid turnover, but contributes significantly to the cleavage of C as expected and also to the amino acids N, Q, S and T.
When combining all four inhibitors, cytosolic AP activity is almost completely abolished, except for a so far unknown AP displaying activity against D, indicating that the four APs characterized in this study account for >95 % of overall cytosolic AP activity.
Discussion
In this work, we present a novel, inhibitor-based approach to create a census of the cytosolic AP activities. Using this method, we identified the major cytosolic AP activities, PSA, BH and two novel players, LTA4H and AP-B, which are responsible for 95 % of total cytosolic AP activity. Additionally, we quantitatively characterized their participation in overall amino acid turnover in this compartment.
In recent years, the cleavage patterns of constitutive proteasomes and immunoproteasomes and their influence on epitope generation and immunodominance have been intensely studied [16–18]. However, APs both in the ER and in the cytosol contribute substantially to the outcome of these epitope precursors. Whereas detailed information about ER-resident APs has been generated [11, 19, 20], the contribution and different specificities of cytosolic APs remain largely unknown. Our experiments provide the first detailed analysis of cytosol-resident APs, their specificities and quantitative impact on N-terminal residue cleavage.
As antigen processing for epitope presentation on MHC class I molecules can be regarded as a byproduct of intracellular protein degradation, the most active proteases should have the highest impact on the presentation of epitopes in health and disease. This assumption is supported by the fact that PSA, which has been shown to be one of the major AP activities, has been described as a trimming peptidase for MHC I epitopes by several groups [21, 22]. PSA (EC 3.4.11.-) is classified as a zinc-dependent AP of the M1 family of metallopeptidases. The human enzyme exists as a monomer in the cytosol and nucleus of cells and is associated with microtubuli. It has been described as neuropeptide regulator and plays a role in the degradation of enkephalin [23]. Of major interest for our work is the participation of PSA in MHC I antigen processing. Here, it has shown to have an impact of the presentation on several epitopes [21, 24]. BH (EC 3.4.22.40), on the other hand, is classified as a member of the C1 family of cysteine proteases. This AP exists as homohexamer in the cytosol and is able to inactivate the drug Bleomycin by hydrolysis [25, 26]. It has been postulated that BH could have a role not only in protein turnover, but also in trimming of epitopes for MHC I antigen processing [24]. Inhibitory studies performed here show the vast impact of PSA and BH on total turnover of amino acids in the cytosol (Fig. 1d), leaving only a residual activity which is composed of enzymatic activity of AP-B and to a smaller extent that of LTA4H.
Like PSA, AP-B (EC 3.4.11.6) is classified as zinc-dependent metalloprotease and member of the M1 family. It is a ubiquitously expressed AP, which selectively cleaves N-terminal R and K residues of peptide substrates. Therefore, a role in epitope trimming events in the cytosol has been proposed [27, 28].
This specificity is in agreement with the activity found with purified cytosolic extracts in this work, but we additionally detected a so far not described preference for P. LTA4H (EC 3.3.2.6), which, according to our observation in this work, has a rather subordinate role in cytosolic turnover, is also a zinc-dependent metalloprotease and again member of the M1 family. It is widely expressed in different tissues [29] and so far was not described in the context of peptide degradation, although it is known to possess AP activity [30, 31]. First and foremost, the enzyme is involved in the formation of the leukotriene LTB4 from arachidonic acid. LTB4 is a potent chemoattractant for neutrophils and hence involved in inflammation as well as in allergy and asthma [32]. LTA4H expression is dependent of the type of immune response, that is, the Th1 cytokine IFN-γ significantly enhances LTA4H mRNA expression, whereas the Th2 cytokines IL-4 und IL-13 decrease LTA4H expression [33]. This is in agreement with the observation that many proteins involved in antigen processing of MHC class I like TAP and the ER-resident aminopeptidase ERAP1 are upregulated by IFN-γ [34].
The combined activity of the four major aminopeptidases, PSA, BH, AP-B and LTA4H, described above almost completely account for total cytosolic AP activity. However, we also provide evidence for an additional yet undefined AP, which we postulate to be specific for the removal of N-Terminal D residues (Fig. 5). The most likely candidate is the Aspartyl Aminopeptidase (EC 3.4.11.21), a cytosolic metallo-aminopeptidase and member of the M18 family. The enzyme displays specificity for D and E with a preference for D [35]. Another previously described AP involved in antigen processing which was not detected in our approach is leucyl aminopeptidase (LAP, EC 3.4.11.1). This is most likely due to the selection of substrates used for screening of cytosolic extracts and chromatographic fractions in this study [36].
Rather than examining the influence of APs on the cell surface expression of specific epitopes, we paid special attention to the cooperation of relevant enzymes and their contribution of total amino acid turnover. Taken together, our quantitative description of the cytosolic AP activity provides the first dataset for modeling N-terminal epitope trimming events in the cytosol. This will facilitate subsequent prediction of N-terminal trimming of proteasomal hydrolysis products, thereby quantitatively defining the pool of potential epitopes available for transport by TAP.
This will for the first time allow to include N-terminal sequence information upstream of the mature epitope into combined prediction algorithms for antigen processing and presentation [37] and also to predict the relevant APs responsible for cytosolic trimming of MHC class I epitopes.
While cytosolic trimming events have been described to impact the pool of peptides available for presentation, the final trimming events and shaping of the peptide repertoire takes place in the ER and is performed by the APs ERAP1 and ERAP2. Other relevant parameters for the prediction of epitope presentation like proteasomal cleavage, TAP affinity of epitope precursors and anchor positions of MHC I molecules are already well established and are used in “reverse immunology” approaches [38]. Future studies investigating the influence of cytosolic and ER-resident proteolytic steps (i.e. additional analysis with physiological peptide substrates) could improve the prediction of immunodominant epitopes in vaccination trials and multiepitope vaccine development. Additionally, specific inhibition of APs by RNA interference or specific protease inhibitors could selectively impact epitope generation to influence either generation or destruction of epitopes. Furthermore, as the proteasome inhibitor Bortezomib was approved in 2004 for the treatment of multiple myeloma, medical applications and prospects of defined AP inhibitors should be considered.
References
The references marked with an asterisk result from the work within project part E6 of the collaborative research center (SFB) 490
Flutter B, Gao B (2004) MHC class I antigen presentation—recently trimmed and well presented. Cell Mol Immunol 1:22–30
Shastri N, Cardinaud S, Schwab SR, Serwold T, Kunisawa J (2005) All the peptides that fit: the beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol Rev 207:31–41
Sijts EJ, Kloetzel PM (2011) The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol Life Sci 68:1491–1502
Doytchinova IA, Flower DR (2006) Class I T-cell epitope prediction: improvements using a combination of proteasome cleavage, TAP affinity, and MHC binding. Mol Immunol 43:2037–2044
Yewdell JW, Reits E, Neefjes J (2003) Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat Rev Immunol 3:952–961
Reits E, Griekspoor A, Neijssen J, Groothuis T, Jalink K, van Veelen P, Janssen H, Calafat J, Drijfhout JW, Neefjes J (2003) Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity 18:97–108
Seifert U, Maranon C, Shmueli A, Desoutter JF, Wesoloski L, Janek K, Henklein P, Diescher S, Andrieu M, de la Salle H, Weinschenk T, Schild H, Laderach D, Galy A, Haas G, Kloetzel PM, Reiss Y, Hosmalin A (2003) An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat Immunol 4:375–379
York IA, Mo AX, Lemerise K, Zeng W, Shen Y, Abraham CR, Saric T, Goldberg AL, Rock KL (2003) The cytosolic endopeptidase, thimet oligopeptidase, destroys antigenic peptides and limits the extent of MHC class I antigen presentation. Immunity 18:429–440
Towne CF, York IA, Neijssen J, Karow ML, Murphy AJ, Valenzuela DM, Yancopoulos GD, Neefjes JJ, Rock KL (2008) Puromycin-sensitive aminopeptidase limits MHC class I presentation in dendritic cells but does not affect CD8 T cell responses during viral infections. J Immunol 180:1704–1712
Towne CF, York IA, Watkin LB, Lazo JS, Rock KL (2007) Analysis of the role of bleomycin hydrolase in antigen presentation and the generation of CD8 T cell responses. J Immunol 178:6923–6930
Haroon N, Inman RD (2010) Endoplasmic reticulum aminopeptidases: biology and pathogenic potential. Nat Rev Rheumatol 6:461–467
*Schatz MM, Peters B, Akkad N, Ullrich N, Martinez AN, Carroll O, Bulik S, Rammensee HG, van Endert P, Holzhutter HG, Tenzer S, Schild H (2008) Characterizing the N-terminal processing motif of MHC class I ligands. J Immunol 180:3210–3217
Schwartz DR, Homanics GE, Hoyt DG, Klein E, Abernethy J, Lazo JS (1999) The neutral cysteine protease bleomycin hydrolase is essential for epidermal integrity and bleomycin resistance. Proc Natl Acad Sci U S A 96:4680–4685
Osada T, Ikegami S, Takiguchi-Hayashi K, Yamazaki Y, Katoh-Fukui Y, Higashinakagawa T, Sakaki Y, Takeuchi T (1999) Increased anxiety and impaired pain response in puromycin-sensitive aminopeptidase gene-deficient mice obtained by a mouse gene-trap method. J Neurosci 19:6068–6078
Walter P, Blobel G (1983) Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol 96:84–93
Goldberg AL, Cascio P, Saric T, Rock KL (2002) The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol 39:147–164
Kincaid EZ, Che JW, York I, Escobar H, Reyes-Vargas E, Delgado JC, Welsh RM, Karow ML, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rock KL (2011) Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat Immunol 13:129–135
*Tenzer S, Wee E, Burgevin A, Stewart-Jones G, Friis L, Lamberth K, Chang CH, Harndahl M, Weimershaus M, Gerstoft J, Akkad N, Klenerman P, Fugger L, Jones EY, McMichael AJ, Buus S, Schild H, van Endert P, Iversen AK (2009) Antigen processing influences HIV-specific cytotoxic T lymphocyte immunodominance. Nat Immunol 10:636–646
Kochan G, Krojer T, Harvey D, Fischer R, Chen L, Vollmar M, von Delft F, Kavanagh KL, Brown MA, Bowness P, Wordsworth P, Kessler BM, Oppermann U (2011) Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-terminal peptide trimming. Proc Natl Acad Sci U S A 108:7745–7750
Saric T, Chang SC, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL (2002) An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat Immunol 3:1169–1176
Levy F, Burri L, Morel S, Peitrequin AL, Levy N, Bachi A, Hellman U, Van den Eynde BJ, Servis C (2002) The final N-terminal trimming of a subaminoterminal proline-containing HLA class I-restricted antigenic peptide in the cytosol is mediated by two peptidases. J Immunol 169:4161–4171
Kim E, Kwak H, Ahn K (2009) Cytosolic aminopeptidases influence MHC class I-mediated antigen presentation in an allele-dependent manner. J Immunol 183:7379–7387
Thompson MW, Hersh LB (2003) Analysis of conserved residues of the human puromycin-sensitive aminopeptidase. Peptides 24:1359–1365
Stoltze L, Schirle M, Schwarz G, Schroter C, Thompson MW, Hersh LB, Kalbacher H, Stevanovic S, Rammensee HG, Schild H (2000) Two new proteases in the MHC class I processing pathway. Nat Immunol 1:413–418
Akiyama S, Ikezaki K, Kuramochi H, Takahashi K, Kuwano M (1981) Bleomycin-resistant cells contain increased bleomycin-hydrolase activities. Biochem Biophys Res Commun 101:55–60
Sebti SM, Lazo JS (1988) Metabolic inactivation of bleomycin analogs by bleomycin hydrolase. Pharmacol Ther 38:321–329
Balogh A, Cadel S, Foulon T, Picart R, Der Garabedian A, Rousselet A, Tougard C, Cohen P (1998) Aminopeptidase B: a processing enzyme secreted and associated with the plasma membrane of rat pheochromocytoma (PC12) cells. J Cell Sci 111(Pt 2):161–169
Foulon T, Cadel S, Cohen P (1999) Aminopeptidase B (EC 3.4.11.6). Int J Biochem Cell Biol 31:747–750
Izumi T, Shimizu T, Seyama Y, Ohishi N, Takaku F (1986) Tissue distribution of leukotriene A4 hydrolase activity in guinea pig. Biochem Biophys Res Commun 135:139–145
Minami M, Ohishi N, Mutoh H, Izumi T, Bito H, Wada H, Seyama Y, Toh H, Shimizu T (1990) Leukotriene A4 hydrolase is a zinc-containing aminopeptidase. Biochem Biophys Res Commun 173:620–626
Habib GM, Cuevas AA, Barrios R, Lieberman MW (1999) Mouse leukotriene A4 hydrolase is expressed at high levels in intestinal crypt cells and splenic lymphocytes. Gene 234:249–255
Haeggstrom JZ, Kull F, Rudberg PC, Tholander F, Thunnissen MM (2002) Leukotriene A4 hydrolase. Prostaglandins Other Lipid Mediat 68–69:495–510
Montero A, Nassar GM, Uda S, Munger KA, Badr KF (2000) Reciprocal regulation of LTA(4) hydrolase expression in human monocytes by gamma-interferon and interleukins 4 and 13: potential relevance to leukotriene regulation in glomerular disease. Exp Nephrol 8:258–265
Zhou F (2009) Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol 28:239–260
Wilk S, Wilk E, Magnusson RP (1998) Purification, characterization, and cloning of a cytosolic aspartyl aminopeptidase. J Biol Chem 273:15961–15970
Matsui M, Fowler JH, Walling LL (2006) Leucine aminopeptidases: diversity in structure and function. Biol Chem 387:1535–1544
*Tenzer S, Peters B, Bulik S, Schoor O, Lemmel C, Schatz MM, Kloetzel PM, Rammensee HG, Schild H, Holzhutter HG (2005) Modeling the MHC class I pathway by combining predictions of proteasomal cleavage, TAP transport and MHC class I binding. Cell Mol Life Sci 62:1025–1037
Lundegaard C, Lund O, Buus S, Nielsen M (2010) Major histocompatibility complex class I binding predictions as a tool in epitope discovery. Immunology 130:309–318
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Akkad, N., Schatz, M., Dengjel, J. et al. Census of cytosolic aminopeptidase activity reveals two novel cytosolic aminopeptidases. Med Microbiol Immunol 201, 463–473 (2012). https://doi.org/10.1007/s00430-012-0266-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-012-0266-x