Abstract
Introduction
Even in the era of proteasome inhibitors and immunomodulatory drugs, the autologous stem cell transplantation after high-dose melphalan continues to represent a standard approach for myeloma patients in first-line therapy. Different mobilization chemotherapies before stem cell apheresis have been published while cyclophosphamide at a dose level of up to 4 g/m2 has been evaluated and is commonly applied. In contrast, lower dose levels of cyclophosphamide (e.g., 1.5 g/m2) did not result in a sufficient collection of stem cells.
Methods
We retrospectively analyzed the impact of “intermediate-dose” (ID-CY, 2.5 g/m2) versus “high-dose” (HD-CY, 4 g/m2) cyclophosphamide in 101 (48 vs. 53) consecutively evaluable myeloma patients (median age 59 years, range 32–72 years) who underwent stem cell mobilization after induction chemotherapy. Successful stem cell harvest was defined as a stem cell yield of at least 5 million CD34 cells per kg bodyweight. Evaluation of toxicity especially considered infectious complications and hematological toxicity in both subgroups.
Results
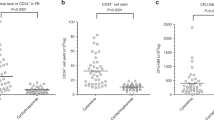
Successful stem cell mobilization was achieved in 40 of 48 (83 %) and 44 of 53 (83 %) patients, respectively. The median time to apheresis (11 vs. 12 days) and the median CD34 content of stem cell harvest (8.3 vs. 7.6 million CD34 cells per kg bodyweight) did not differ significantly between both groups. There was a significant difference of WBC nadir in favor of the cyclophosphamide regimen with 2.5 g/m2 (0.8 vs. 0.3 Gpt/L, p = 0.021), and neutropenic fever was more often observed in patients who received 4 g/m2 cyclophosphamide (34 vs. 15 %, p = 0.078). Importantly, after induction chemotherapy using the VCD regimen (bortezomib, cyclophosphamide, dexamethasone), successful stem cell mobilization was achieved in 26 of 29 (90 %) patients treated with 2.5 g/m2 and 21 of 25 (84 %) patients receiving 4 g/m2 cyclophosphamide, respectively.
Conclusions
ID-CY is safe and highly effective for stem cell mobilization in patients with newly diagnosed myeloma and associated with a reduced toxicity compared to HD-CY.
Similar content being viewed by others
References
Afifi S, Michael A, Lesokhin A (2016) Immunotherapy: a new approach to treating multiple myeloma with daratumumab and elotuzumab. Ann Pharmacother 50:555–568
Anderson KC, Alsina M, Atanackovic D et al (2016) NCCN guidelines insights: multiple Myeloma, Version 3.2016. J Natl Compr Canc Netw 14:389–400
Antar A, Otrock ZK, Kharfan-Dabaja MA et al (2015) G-CSF plus preemptive plerixafor vs hyperfractionated CY plus G-CSF for autologous stem cell mobilization in multiple myeloma: effectiveness, safety and cost analysis. Bone Marrow Transplant 50:813–817
Attal M, Harousseau JL, Stoppa AM et al (1996) A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 335:91–97
Awan F, Kochuparambil ST, Falconer DE et al (2013) Comparable efficacy and lower cost of PBSC mobilization with intermediate-dose cyclophosphamide and G-CSF compared with plerixafor and G-CSF in patients with multiple myeloma treated with novel therapies. Bone Marrow Transplant 48:1279–1284
Cavallo F, Bringhen S, Milone G et al (2011) Stem cell mobilization in patients with newly diagnosed multiple myeloma after lenalidomide induction therapy. Leukemia 25:1627–1631
Cavo M, Palumbo A, Zweegman S et al (2016) Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): a randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). ASCO Meet Abstr 34:8000
Child JA, Morgan GJ, Davies FE et al (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875–1883
Deliliers GL, Annaloro C, Marconi M et al (2002) Harvesting of autologous blood stem cells after a mobilising regimen with low-dose cyclophosphamide. Leuk Lymphoma 43:1957–1960
Fitoussi O, Perreau V, Boiron JM et al (2001) A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant 27:837–842
Gay F, Oliva S, Petrucci MT et al (2015) Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol 16:1617–1629
Giralt S, Costa L, Schriber J et al (2014) Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant 20:295–308
Hamadani M, Kochuparambil ST, Osman S et al (2012) Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant 18:1128–1135
Hartmann T, Hubel K, Monsef I et al (2015) Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev. doi:10.1002/14651858.CD010615.pub2
Jillella AP, Ustun C, Robach E et al (2003) Infectious complications in patients receiving mobilization chemotherapy for autologous peripheral blood stem cell collection. J Hematother Stem Cell Res 12:155–160
Kropff M, Liebisch P, Knop S et al (2009) DSMM XI study: dose definition for intravenous cyclophosphamide in combination with bortezomib/dexamethasone for remission induction in patients with newly diagnosed myeloma. Ann Hematol 88:1125–1130
Kumar S, Giralt S, Stadtmauer EA et al (2009) Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood 114:1729–1735
Kumar SK, Ma E, Engebretson AE et al (2016) Treatment outcomes, health-care resource utilization and costs of bortezomib and dexamethasone, with cyclophosphamide or lenalidomide, in newly diagnosed multiple myeloma. Leukemia 30:995–998
Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23:3–9
Mai EK, Bertsch U, Durig J et al (2015) Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia 29:1721–1729
Manier S, Barthelemy M, Fouquet G et al (2012) Reduced steady state-based peripheral blood stem cell harvest rate in multiple myeloma treated with bortezomib-based induction regimens. Leukemia 26:2552–2554
Mohty M, Hubel K, Kroger N et al (2014) Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 49:865–872
Moreau P, Hulin C, Macro M et al (2016) VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood 127:2569–2574
Oakervee HE, Popat R, Curry N et al (2005) PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol 129:755–762
Perea G, Sureda A, Martino R et al (2001) Predictive factors for a successful mobilization of peripheral blood CD34+ cells in multiple myeloma. Ann Hematol 80:592–597
Reeder CB, Reece DE, Kukreti V et al (2009) Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 23:1337–1341
Sheppard D, Bredeson C, Allan D, Tay J (2012) Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant 18:1191–1203
Sureda A, Bader P, Cesaro S et al (2015) Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant 50:1037–1056
Sutherland DR, Anderson L, Keeney M et al (1996) The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother 5:213–226
Tandon N, Ramakrishnan V, Kumar SK (2016) Clinical use and applications of histone deacetylase inhibitors in multiple myeloma. Clin Pharmacol 8:35–44
Terpos E, Kleber M, Engelhardt M et al (2015) European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica 100:1254–1266
Toor AA, van Burik JA, Weisdorf DJ (2001) Infections during mobilizing chemotherapy and following autologous stem cell transplantation. Bone Marrow Transplant 28:1129–1134
Tuchman SA, Bacon WA, Huang LW et al (2015) Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher 30:176–182
Acknowledgments
We thank the Institute of Clinical Chemistry and Laboratory Medicine of the University Hospital Jena, Germany, for the support in routine diagnostic concerning the measurement of CD34-positive cells in peripheral blood and stem cell grafts of our patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Winkelmann, N., Desole, M., Hilgendorf, I. et al. Comparison of two dose levels of cyclophosphamide for successful stem cell mobilization in myeloma patients. J Cancer Res Clin Oncol 142, 2603–2610 (2016). https://doi.org/10.1007/s00432-016-2270-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2270-9