Abstract
In the last decades, in particular forest ecosystems became increasingly N saturated due to elevated atmospheric N deposition, resulting from anthropogenic N emission. This led to serious consequences for the environment such as N leaching to the groundwater. Recent efforts to reduce N emissions raise the question if, and over what timescale, ecosystems recover to previous conditions. In order to study the effects on N distribution and N transformation processes under the lowered N deposition treatment, we investigated the fate of deposited NH4 +-15N in soil of a N-saturated Norway spruce forest (current N deposition: 34 kg ha−1 year−1; critical N load: 14 kg ha−1 year−1), where N deposition has been reduced to 11.5 kg ha−1 year−1 since 14.5 years. We traced the deposited 15N in needle litter, bulk soil, and amino acids, microbial biomass and inorganic N in soil. Under reduced N deposition, 123 ± 23% of the deposited N was retained in bulk soil, while this was only 72 ± 15% under ambient deposition. We presume that with reduced deposition the amount of deposited N was small enough to become completely immobilized in plant and soil and no leaching losses occurred. Trees receiving reduced N deposition showed a decline in N content as well as in 15N incorporation into needle litter, indicating reduced N plant uptake. In contrast, the distribution of 15N within the soil over active microbial biomass, microbial residues and inorganic N was not affected by the reduced N deposition. We conclude that the reduction in N deposition impacted only plant uptake and drainage losses, while microbial N transformation processes were not influenced. We assume changes in the biological N turnover to start with the onset of the decomposition of the new, N-depleted litter.
Similar content being viewed by others
Introduction
The global nitrogen (N) deposition increased by a factor of four since the middle of the twentieth century due to increasing anthropogenic activities, like fossil fuel combustion or emissions from agricultural land (Galloway et al. 2004). As a result, naturally N-limited forest ecosystems became increasingly N saturated, since N deposition exceeded the biological demand and the storage capacity of soil (Aber et al. 1989). This causes N losses to the atmosphere and aquatic systems, resulting in acidification and eutrophication of aquatic and terrestrial ecosystems (Galloway et al. 2004). In consequence, critical loads for the different regions and emission reduction policies were specified, and N deposition rates started to decrease (Fowler et al. 2007). So far, it is unresolved how long N-impacted forest ecosystems may need to recover and how different ecosystem characteristics may change.
Several studies have addressed the effect of atmospheric N reduction to pre-industrial levels in forest ecosystems. Gundersen et al. (1998) reported a fast reduction in N leaching losses under reduced N deposition at N-saturated sites. Lamersdorf and Borken (2004) observed reduced N concentrations in live fine roots and in 1-year-old needles 2 and 4 years after reduced N deposition was initiated. An initial increase in soil solution pH after 1.5 years (Bredemeier et al. 1995a, b) was not confirmed by studies dealing with long-term reduction of N and acid inputs (Lamersdorf and Borken 2004). A laboratory study by Corre and Lamersdorf (2004) showed a slight increase in gross N mineralization and higher microbial ammonium immobilization rates in soils subjected to 10 years of reduced N deposition. For N-reduced plots, they reported shorter mean residence times of ammonium as well as of microbial N along with invariant contents of ammonium and microbial biomass N in the organic layer. No significant differences in turnover rates were observed for the mineral soil (Corre and Lamersdorf 2004).
Studies dealing with reduction of N deposition used 15N to trace the deposited N within ecosystems (distribution over plant biomass, bulk soil, leaching loss; e.g., Koopmans et al. 1996; Tietema et al. 1998). In all cases, more 15N was recovered in soil upon reduced N deposition, thus, indicating increased soil N retention and reduced N leaching losses. Feng et al. (2008) investigated the retention of ammonium from ambient atmospheric deposition and observed higher N incorporation into the soil (71%) rather than into tree biomass (30%). However, most studies have addressed the distribution of 15N over different tree constituents (e.g., needles, twigs, branches, trunks) and not much attention has been given to N retention and its distribution over different soil constituents, neither under ambient nor under reduced N deposition.
In a previous study we could show that the reduction of N deposition did not affect the concentration and composition of soil organic nitrogen (ON), but initially affects the ecosystem at the plant level (Dörr et al. 2010). A decline in ON in the litter under reduced N deposition was observed but because of the long life-span of needles and the slow degradation of OM under acidic conditions the time period of the N reduction seemed too short to cause alterations in soil N in deeper horizons. Therefore, we took advantage of the 15N tracer applied since 2001 (10 years after the start of the N reduction). The 15N enables tracing of deposited ammonium in the ecosystem, thus to track its distribution within the soil. The long-term application ensures incorporation of 15N at measurable concentrations within all important ecosystem compartments, including plants and microbial residues, at the disadvantage of a cross labeling by 15N-enriched litter and by N recycling in soil.
We aimed to investigate the response of the soil–plant system to 14.5 years of reduced N deposition. Our objectives were to (I) quantify the deposited N that is retained in soil, (II) determine the distribution of deposited N over inorganic N species, active microbial biomass, amino acids (representing microbial residues and the main part of ON), and needle litter (representing recent plant biomass and the source material for cross labeling via N mineralization), and (III) assess effects of the reduced N deposition on N distribution over and incorporation into different soil constituents, that were not detectable by analyzing only content and composition of soil ON. We hypothesized larger 15N recoveries in needle litter as well as more pronounced 15N retention in soil under reduced N deposition because of N limitation and, thus, changes in the 15N distribution over the different soil constituents.
Materials and methods
Site characterization
The site is located on the Solling plateau in central Germany (51°31′N, 9°34′E), at an elevation of 500 m above sea level. The area is characterized by a temperate sub-oceanic climate with a mean annual air temperature of 6.4°C and an annual precipitation of 1,090 mm. The soils are Dystric Cambisols (FAO 1998), developed from a loess solifluction layer overlaying sandstone bedrock. The soil texture is silty loam and the organic layer is moder-type. Exchangeable cations (Mg, K, Ca, Na) and acidity strongly decreased down the profile of the mineral soil, with the proportion of exchangeable cations remaining constant. Initial podzolization is indicated by reversal depth distribution of poorly crystalline Fe and Al (hydr)oxides within the mineral soil. For details, see Dörr et al. (2010).
Experimental design
The experimental site was established in 1989 in a currently (2010) 76-years-old Norway spruce (Picea abies (L.) Karst.) stand (Bredemeier et al. 1998). The experimental setup consists of four different plots, one ‘ambient no-roof’ plot, to quantify possible roof effects, and three treatment plots (‘ambient roof’, ‘clean rain’ and ‘ambient roof no-label’), each covered with transparent roofs (each 300 m2) below the canopy of the forest and 3.5 m above the ground.
The N treatment started in September 1991. Throughfall water is permanently collected from the roof, filtered (<350 μm) to remove organic debris and re-sprinkled immediately without deionization (‘ambient roof’ plot: 34 ± 1 kg N ha−1 year−1) or after partial deionization (‘clean rain’ plot: 11.5 kg N ha−1 year−1; Corre and Lamersdorf 2004). The ‘ambient no-roof’ plot is exposed to recent throughfall conditions with a mean N deposition of 33 ± 2 kg ha−1 year−1 (Corre and Lamersdorf 2004).
The application of 15N started in November 2001. Using additional sprinkler systems (~80 cm above the ground), the 15NH4 + (95 atom%, applied as 15NH4NO3) was added to the throughfall water separately as a 1-mm rain event after every 30–40 mm regular throughfall at the ‘clean rain’ and ‘ambient roof’ plot. Table 1 shows 15N application amounts from November 2001 to September 2008. The treatment aimed at applying comparable relative amounts of 15N [% N deposition] to both plots. The ‘ambient roof no-label’ served as a control for the labeling experiment exposed to equal N deposition like the ‘ambient roof’ plot, but without 15N addition.
In April 2006, each treatment plot was divided into four subplots, which were individually sampled. At each subplot, five replicate soil core were extracted and sectioned by horizon (Oe, Oa, A, Bw). Soil material of the same horizon was combined and air dried. Mineral soil samples were sieved to <2 mm. Litter, which accumulates on the roofs, is re-distributed on the plots 4–5 times per year, and was therefore only used for source material characterization. Hence, field replications of Oi horizons (litter) were pooled and not statistically evaluated.
Soil cores for extraction of microbial biomass were sampled fresh in March, June, and September 2008. From each subplot, two soil cores were sectioned by horizon (Oi, Oe, Oa, A, Bw), pooled and stored at 4°C prior to measurements (maximal storage time: 5 days).
15N abundance and N content in different soil constituents
Ammonium and nitrate
Inorganic N was extracted with 1 M KCl at a soil-to-solution ratio of 1:10 for organic layers and 1:5 for mineral soil material. Content and 15N abundance of NH4 + as well as of NO3 − were determined by Sample Preparation unit for Inorganic Nitrogen coupled to a Mass Spectrometer (SPINMAS) according to Stange et al. (2007). While NH4 + was measured as N2 after oxidation with NaOBr in NaOH, NO3 − was reduced to NO by V(III)Cl3 in HCl. As also NO2 − was reduced to NO by V(III)Cl3, the formed NO represents a mixture of both N-species. In soil solutions NO3 − predominates over NO2 −, hence we did not correct for NO2 −. 15N abundances were expressed as atom%.
Microbial biomass
Microbial biomass N was determined by the chloroform fumigation–extraction method (Brookes et al. 1985), modified according to Lipson and Monson (1998). After determination of soil dry mass, each sample was divided into two subsamples. The first subsample was fumigated with chloroform in the dark at 25 ± 2°C for 24 h and then extracted with distilled water (soil-to-solution ratio: organic layer = 1:20, mineral soil = 1:5). The second subsample was used as control, thus extracted with distilled water without further treatment. Extracts were kept frozen until analyzing for total N by a total organic carbon analyzer (TOC-V CSH, Shimadzu Corp., Tokyo, Japan), equipped with a total nitrogen measuring unit (TNM-1, Shimadzu Corp.). Soil microbial biomass N (MB-N) was calculated as the difference in total N between fumigated and unfumigated extracts, divided by the factor of extractability of microbial biomass N (K en). According to Nordin et al. (2004) we used a K en factor of 0.45, because of the 15–20% lower extractability of N in water than in K2SO4 (K en = 0.54; Brookes et al. 1985).
The remainder of the extracts was frozen, freeze dried and subsequently analyzed for 15N by isotope ratio mass spectrometry (IRMS), using an elemental analyzer (Euro EA, Hekatech GmbH, Wegberg, Germany) coupled to a Delta Advantage IRMS (Thermo Scientific, Bremen, Germany).
15N values were expressed as δ15N, which represents the ratio of 15N to 14N relative to the atmospheric standard (R standard = 0.0036765, corresponding to 0.3663 atom%; Högberg 1997), expressed as per mill (Eq. 1):
where R is the ratio of the heavy isotope (15N) to the light one (14N). The 15N abundance in microbial biomass was calculated according to 15N mass balance (Eq. 2; Ryan et al. 1995):
where Nf and Nu are the total N extracted from the fumigated and the unfumigated subsample, and MBδ15N, δ15Nf and δ15Nu are the 15N abundances [‰] of the microbial biomass, the fumigated extract and the unfumigated extract.
Amino acids
Hydrolysable amino acids (HAA) were extracted from ground soil (<200 μm), and quantified as the sum of enantiomers of individual amino acids following the method of Amelung and Zhang (2001). Briefly, the amino acids were hydrolyzed with 6 M HCl for 12 h at 105°C, purified by cation exchange resin (DOWEX 50 W × 8), and converted into N-pentaflouropropionyl-isopropyl esters, which were determined by gas chromatography–mass spectrometry (GCMS-QP2010; Shimadzu Corp., Tokyo, Japan). For details of analysis, see Dörr et al. (2010).
Compound-specific stable isotope analyzes of 15 amino acids (Ala: alanine, Val: valine, Thr: threonine, Gly: glycine, Ile: isoleucine, Pro: proline, Leu: leucine, Ser: serine, Asp: aspartic acid, Glu: glutamic acid, Met: methionine, Phe: phenylalanine, Tyr: tyrosine, Lys: lysine and norvaline) was carried out on a gas chromatograph (Trace GC 2000; Thermo Finnigan, Bremen, Germany), coupled to an isotope ratio mass spectrometer (Delta PlusTM; Thermo Finnigan) via a combustion interface (GC combustion III, Thermo Finnigan; GC-C-IRMS). The N-pentaflouropropionyl-isopropyl esters of the extracted amino acids were separated according to Sauheitl et al. (2009).
Since no certified calibration standard is available for GC-C-IRMS measurements, the stable N isotope compositions of standard amino acids was calibrated against certified standards by EA-IRMS analysis (Carlo Erba NC 2500; Carlo Erba Instruments, Milan, Italy, coupled to a Delta PlusTM IRMS; Thermo Finnigan) and used to correct for systematic differences between EA- and GC-C-IRMS measurements.
15N values were expressed as δ15N (Eq. 1). The 15N abundance of total hydrolysable amino acids (THAA) was calculated according to 15N mass balance (Eq. 3):
where HAAδ15N and HAA-N are the 15N abundances [‰] and hydrolysable amino acid N contents [μg g−1 dry mass soil] of each amino acid, respectively, and THAAδ15N and THAA-N of the sum of amino acids.
Bulk soil and needle litter
Bulk stable N isotope composition of each horizon (Oi, Oe, Oa, A, Bw) and of needles sampled above the roof, thus having not received 15N via deposition, was carried out on ground samples with a EA-IRMS system (Euro EA coupled to a Delta Advantage IRMS). 15N abundances were expressed as δ15N according to Eq. 1.
15N content and recovery in soil constituents
The mass of 15N recovered in individual soil constituents of the labeled plots was calculated from the 15N mass balance, assuming no net fractionation to be associated with N fluxes through the constituent in question (Eq. 4; Nadelhoffer and Fry 1994):
where 15Nconst is the mass of 15N recovered in labeled soil constituents in [g m−2]; δ15Nconst and δ15Ncontrol are the 15N abundances of soil constituents of the labeled plot (‘clean rain’ or ‘ambient roof’) and the ‘ambient roof no-label’ plot in [‰]; δ 15Ntracer is the 15N abundance of the tracer (95 atom%), which was translated into [‰] according to Eq. 1; Nconst is the mass of N in labeled soil constituents in [g m−2]. Nconst was estimated from the N content and the bulk densities of each horizon or needle litter mass (Feng et al. 2008).
15N recovery of the added tracer in constituents of the two labeled plots was calculated as percentage of total applied 95 atom% 15N tracer in [g N m−2]. 15N excess (∆δ15N) of each constituent was calculated by the difference between 15N abundances of the labeled plots (‘clean rain’, ‘ambient roof’) and the ‘ambient roof no-label’ plot. To compare the 15N distribution between the two labeled plots, we normalized the 15N recoveries for each soil constituent (THAA, MB, NH4 +, NO3 −/NO2 −) to 15N recovered in each bulk soil horizon and in the whole soil profile, respectively.
Statistics
Since 15N application was not replicated, statistical analyses were solely performed on the four replicates within each plot (subplots; i.e., pseudo replicates). The influence of reduced N deposition (‘clean rain’ vs. ‘ambient roof’ plot) on 15N recovery and N content of soil constituents was determined by paired t-tests for each horizon. Effects of soil depth on 15N recovery and N content of soil constituents were tested by one-way analysis of variance (ANOVA) for each plot. The effect of sampling time on N contents in microbial biomass was determined by ANOVA for each horizon and plot. Statistics were applied after testing for normality (Kolmogorov–Smirnov test) and homogeneity of variances (Levene test). The non-parametric Mann–Whitney U-test and the Kruskal–Wallis H-test were used for non-normally distributed data. All calculations were carried out with the software package SPSS (Version 10.0, SPSS Inc., Chicago, USA).
Results
Basic soil properties and total, inorganic and organic N
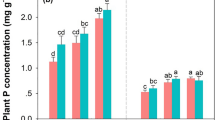
The pH (CaCl2) ranged from 2.6 in Oa to 4.1 in Bw horizons. Organic N (ON) dominated in all analyzed samples, with 98.8 ± 0.2% of total N (TN). Total, organic and inorganic N (IN) decreased significantly with depth (p ≤ 0.01, Table 2). Inorganic N was dominated by ammonium (between 99.1% in the litter and 77.4% in Bw horizon), with minor amounts of nitrate plus nitrite (Table 2). Since the decline with soil depth is more pronounced for OC than for N, C/N ratios decreased significantly with depth (p ≤ 0.01), being highest in the litter and lowest in Bw horizons.
Nitrogen contents were not affected by N treatment throughout the soil profile, except for the litter (Oi) horizon. Inorganic N in the Oi horizon at the ‘clean rain’ plot was in the range of those at plots receiving actual N deposition loads, while TN and ON contents were smaller, and the C/N ratio was widest in the ‘clean rain’ plot (Table 2).
Nitrogen in microbial biomass
Microbial biomass N (MB-N) varied between the three sampling times (Fig. 1). In litter at the ‘clean rain’ and the ‘ambient roof’ plot, MB-N was significantly lower (p ≤ 0.05) in September than in March and June.
The contribution of MB-N to TN significantly decreased with depth (p ≤ 0.05) from the Oi layers (mean: 5.1 ± 0.6% TN; corresponds to 5.4 ± 0.8% ON) to the Bw horizons (mean: 0.44 ± 0.05% TN; corresponds to 0.44 ± 0.05% ON; Fig. 1). Microbial biomass N was not significantly affected by N treatment.
Nitrogen in amino acids
Total amino acid N released upon HCl hydrolysis (THAA-N) decreased significantly with depth (p ≤ 0.05) from the Oe layers (40.4 ± 1.1% TN; corresponds to 41.0 ± 1.2% ON) to the Bw horizons (12.4 ± 0.6% TN; corresponds to 12.4 ± 0.6% ON, Table 3). In litter, THAA-N contributed to 48.5 ± 2.0% to ON (corresponds to 46.5 ± 1.3% TN) and was predominated by N with Lys and Gly, followed by Ala, Leu, Glu, Pro and Asp > Ser > Thr > Val > Phe > Ile > Tyr > Met. Shares of total ON with all hydrolysable amino acids decreased with depth down to the Bw horizons, except for Lys, which increased from A to Bw horizons (Table 3). The decline in N with neutral amino acids (Gly, Ala, Leu, Pro, Ser, Thr, Val, Phe, Ile, Tyr, Met) from A to Bw horizons correlated positively with the N content of the individual amino acids (r = 0.85), while no correlation was found for N with basic (Lys) or acidic amino acids (Asp, Glu). N-normalized amino acid patterns showed a clear horizontal stratification between the upper horizons and the Bw horizons but revealed no N treatment effect for these horizons. Concordantly, also THAA-N was not affected significantly by N deposition.
15N recoveries of the added tracer throughout soil profiles under reduced and ambient deposition
At the ‘ambient roof’ plot, 71.8 ± 15.1% of the added 15N was found in the bulk soil, with the highest recovery in the Oe layer (45.7 ± 8.7%), decreasing significantly (p ≤ 0.01) down to the Bw horizon (3.3 ± 1.4%; Table 4). The ‘clean rain’ plot, where 123.2 ± 23.0% of the added 15N was recovered in the total bulk soil (Table 4), showed the same depth trend. Due to the large standard errors, which are typical for such labelling experiments, differences among the plots were not statistically significant. Inorganic N accounted for only 4.4 ± 0.4% at the ‘ambient roof’ and for 7.3 ± 0.8% at the ‘clean rain’ plot of the recovered 15N (Table 4). Consequently, most of the tracer 15N was incorporated into ON. At both plots, ammonium comprised the major part to the inorganic 15N. THAA contained the largest percentage of the tracer 15N of all analyzed soil constituents (‘ambient roof’: 19.5 ± 4.1%; ‘clean rain’: 31.5 ± 5.9%), with highest values in Oe layers and a significant decrease (p ≤ 0.01) to Oa and deeper horizons at both plots (Table 4). The active MB incorporated on average 7.4 ± 2.2% of the tracer at the ‘ambient roof’ and 11.8 ± 0.9% at the ‘clean rain’ plot and showed the same trend with depth as THAA.
15N recovered with the analyzed soil constituents decreased with depth at both plots. Recoveries in bulk samples, thus, in all soil constituents tended to be larger at the ‘clean rain’ than at the ‘ambient roof’ plot. However, differences were not statistically significant. Needle litter sampled above the roof (5 to 8-year old needles) contained 0.4% of the added 15N at the ‘ambient roof’ plot and showed natural 15N abundance at the ‘clean rain’ plot (Table 4).
15N distribution under reduced and ambient N deposition
In both plots, most 15N was recovered in THAA (‘clean rain’: 27.6 ± 5.4% of bulk soil-15N, ‘ambient roof’: 26.6 ± 1.4% of bulk soil-15N; Table 5), followed by MB and ammonium, with negligible amounts in nitrate plus nitrite. The contribution of THAA, MB and ammonium to bulk soil 15N showed no significant changes throughout the soil profiles (Fig. 2). The 15N distribution of nitrate plus nitrite varied strongly within individual horizons, due to small recoveries, partly below or around the detection level (data not shown). The distribution of 15N in the MB did not vary significantly with sampling time (Fig. 3). Ala, Gly and Glu contained most 15N within the THAA, followed by Leu, Lys, Asp and Pro (Fig. 4). Minor amounts of 15N were found with Thr, Val, Ser, Phe, Ile and Tyr. On average, the ‘clean rain’ and the ‘ambient roof’ plot showed similar percentages of 15N incorporated into individual HAA (0.22–5.42% bulk soil-15N). In both plots, the percentage of 15N incorporation into individual HAA remained constant throughout the entire profile, while the composition of THAA, especially in the mineral soil, changed with depth. Lys increased from the A to Bw horizon at both plots, while Asp and Ser decreased relative to the other HAA at the ‘ambient roof’ (Fig. 4). The 15N distribution in HAA at the ‘clean rain’ plot showed stronger variations, but associated with higher standard errors.
Proportion of 15N recovery in a total hydrolysable amino acids, b microbial biomass and c ammonium on 15N recovered in bulk soil [%] at the ‘clean rain’ (black) and ‘ambient roof’ plot (white). Values given are means and error bars indicate standard errors (n = 4, except total hydrolysable amino acids and NH4 + in the litter)
The 15N distribution into MB, IN and HAA within the soil profile was not influenced by the different N deposition.
Discussion
15N recovery and retention in the ecosystem
Previous studies at the plot receiving ambient N deposition showed that the major part of the 15N tracer added as 15NH4 + was recovered in the soil (71.0%) followed by tree biomass (30.0%), with minor leaching losses (3.8%; Feng et al. 2008). Bredemeier et al. (1995a) reported NH4 + and NO3 − concentrations below the detection level in the soil solution under reduced N deposition since September 1992. We therefore presume no leaching losses of 15N at this plot. The recovery of added 15N in the bulk soil under ambient N deposition (71.8 ± 15.1%; Table 4) fits well the results of Feng et al. (2008). Koopmans et al. (1996) reported that the retention of throughfall N is relatively more important at reduced N deposition levels than at high N deposition. Our results show the same trend with higher 15N recoveries at the plot receiving reduced N deposition (bulk soil: 123.2 ± 23.0%; Table 4). This is also supported by the fact that the N deposition at this plot (11.5 kg N ha−1 year−1) is below the calculated N retention capacity of the soil under ambient N deposition of about 24.4 kg N ha−1 year−1 (equates to 71.8% of the throughfall N) and below the ‘critical load’ of about 14 kg N ha−1 year−1 (de Vries et al. 1995). We can exclude a stronger dilution of 15N via stored 14N in the soil upon ambient N deposition, because of similar soil N contents in both plots (Table 2). We therefore assume the higher 15N recoveries under reduced than under ambient N deposition to be due to the decline in bioavailable N, thus, higher N retention. This indicates that the N deposition exceeds the ecosystem’s N retention capacity at the plot receiving ambient N deposition. Under reduced N deposition, the ecosystem is processing the incoming N more efficiently, without detectable N losses, but also without detectable N deprivation, since N stored in the soil did not change during the 14.5 years of reduced N deposition.
Since the 15N recovery in bulk soil is higher under reduced than under ambient N deposition and differences in 15N leaching losses do not impact much total 15N budgets, we assume lower 15N incorporation into tree biomass under reduced N deposition. In contrast, Nadelhoffer and Fry (1994) assumed complete N uptake when N is limited (e.g., upon reduced input), resulting in no or negligible isotopic fractionation. Koopmans et al. (1996) showed an increase in 15N recovery in a Douglas fir forest due to the strong dependency on throughfall N of the plants at this site, but also a decrease in 15N recovery in a Scots pine forest after reduction of N deposition. They suggested that when ecosystems are N saturated, plants store excess N with free amino acids in foliage, which are utilized primarily after N reduction. The reduced N in needles upon reduced N deposition (Lamersdorf and Borken 2004) and the absence of tracer 15N in needle litter taken above the roof at the plot receiving reduced N deposition (Table 4) support the idea of Koopmans et al. (1996) of an internal storage. Therefore, reduction of the N deposition did not cause N limitation to the Norway spruce forest so far.
In agreement with studies of Tietema et al. (1998) and Feng et al. (2008), most of the added 15N was retained in the organic layers at both study plots (Table 4), indicating that the deposited ammonium 15N was incorporated into the organic matter in these horizons. Bulk soil N was predominated by ON, which went along with higher 15N recoveries in organic compared to inorganic N.
15N distribution in relation to N deposition
The distribution of 15N within the soil was not changed by the reduction of N deposition (Table 5). The two plots, receiving reduced and ambient N deposition, showed similar 15N distribution over the soil constituents (THAA, MB and IN species) throughout all soil horizons (Fig. 2), indicating that the microbial community and activity did not change during the 14.5 years under reduced N deposition. This is also supported by the lack in deprivation of bioavailable N (Table 2).
Amino acid N represented the predominant fraction of ON in the study soils and showed no response to reduced N deposition (Dörr et al. 2010). Castro et al. (2006) showed that most tracer 15N incorporated into organic matter is recovered with unidentified hydrolysable N, immediately followed by amino acid N and by amide N; little 15N is recovered with amino sugar N. The predominating HAA (Ala, Gly, Glu, Leu, Lys, Pro, Asp) showed highest 15N recoveries (Fig. 4), indicating that they are most frequently recycled. With depth, concentrations of HAA-N decreased relative to TN (Table 3), while 15N recoveries normalized to 15N recovered in bulk soil remained constant (Fig. 4). This indicates losses due to progressing and preferential degradation of organic compounds enriched in 14N. The only exceptions were Lys, which showed an increase in 15N recovery as well as in concentration from A to Bw horizon, and Asp, with a decrease in content and 15N recovery within the mineral soil (Fig. 4). Furthermore, the decrease of neutral amino acids from A to Bw horizon correlated well with their N contents, while we found no relation for basic (Lys) and acidic amino acids (Asp, Glu). These results indicate that neutral amino acids were used preferentially as N sources during degradation. In turn, the increase of Lys and the relatively stronger decrease of Asp seem to depend on differential leaching and stabilization, as caused by their associations with different organic compounds (Dörr et al. 2010).
The 15N recoveries with MB (8.7–10.9%; Table 5) of the studied long-term labeling experiment are small compared to short-term experiments (16–49%; Emmett and Quarmby 1991; Nordin et al. 2004). This is likely due to the changing composition of 15N in bulk soil with time. The MB continuously incorporates deposited 15N. After death, 15N-enriched microbial residues (e.g., amino acids, amino sugars) become stabilized by association with organic compounds or mineral phases, and accumulate with time. Concentrations of MB-N, in proportion to TN, decreased from the Oi down to Bw horizon (Fig. 1), while 15N recoveries normalized to 15N recovered in bulk soil remained constant (Fig. 2). This indicates increasing relative contribution of 15N-enriched OM with soil depth. Tietema et al. (1998) concluded that microbial population and drainage losses are first affected by changed N inputs. In contrast, Koopmans et al. (1996) reported incorporation of about 10% of the deposited 15NH4 + into microbial biomass within 1 year, independent of the N input. Concordantly, we found no effect of the N deposition on the 15N incorporation into MB as well as into amino acids.
Nitrogen transformation processes
The long-term labeling via 15NH4 + includes the problem of recycling, where degradation of organic compounds containing previously incorporated 15N may result in labeled secondary metabolites. Therefore, 15N in certain soil constituents did not solely result from direct N immobilization. Nevertheless, we can exclude cross labeling via 15N-containing litter since needles taken above the roof (receiving no 15NH4 +) showed no (under reduced N deposition) or negligible 15N (under ambient N deposition: 0.23 mg m−2; equates to 0.42% of added 15N; Table 4). Furthermore, the predominating portion of C in the Oe horizon has been assimilated in 1984–1985 (Dörr et al. 2010) and the new litter (enriched in 15N) has not been incorporated into deeper organic layers so far; instead it forms the Oi layer.
At both plots, the active MB as well as the microbial residues showed 15N incorporation, indicating that added 15NH4 + was immobilized and microbial N was recycled. Compared to ammonium, nitrate plus nitrite were minor inorganic N species (Table 2), with minor 15N recoveries (Table 4), indicating no or negligible nitrification (Corre and Lamersdorf 2004), resulting from the low pH values.
Previous studies on soil OM at the study site showed that the reduced N deposition neither affected the content of organic C and N compounds nor changed the composition of the microbial community involved in degradation so far (Theuerl et al. 2010; Dörr et al. 2010). Investigations of 15N enable the tracing of potential changes in N transformation processes upon reduced N deposition, as found in a laboratory study (Corre and Lamersdorf 2004). Yet, we did not observe any differences in 15N distribution in soil under ambient and reduced N deposition.
Conclusion
Previous studies revealed that 14.5 years of reduced N deposition resulted in initial effects on the plant level, while content and composition of soil OM did not change, because of the large amount of N stored in the soil and the slow degradation of the new, N-depleted litter. Nevertheless, 15N labeling showed larger retention of deposited ammonium in the soil under reduced atmospheric N input. However, no effects on soil N cycling were observed, as indicated by the similar 15N distribution over active microbial biomass and residues under reduced and ambient N deposition. The study shows that reduction of throughfall N to pre-industrial level results in N input below the soil’s estimated N retention capacity, thus causing reduced N losses. However, it did not result in a shortage of bioavailable N and so stored organic N was not microbially mobilized. Obviously, the reduction in N deposition by 65% did not cause N limitations to microbial processes. We presume first changes in N cycling in the soil, along with changes in the microbial decomposer community, to occur upon the beginning of the degradation of the new, N-depleted litter.
Abbreviations
- HAA:
-
Hydrolysable amino acids
- IN:
-
Inorganic nitrogen
- MB:
-
Microbial biomass
- OC:
-
Organic carbon
- OM:
-
Organic matter
- ON:
-
Organic nitrogen
- THAA:
-
Total hydrolysable amino acids
- TN:
-
Total nitrogen
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM (1989) Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–386
Amelung W, Zhang X (2001) Determination of amino acid enantiomers in soils. Soil Biol Biochem 33:553–562
Bredemeier M, Blanck K, Lamersdorf N, Wiedey GA (1995a) Response of soil water chemistry to experimental ‘clean rain’ in the NITREX roof experiment at Solling, Germany. For Ecol Manag 71:31–44
Bredemeier M, Dohrenbusch A, Murach D (1995b) Response of soil water chemistry and fine-roots to clean rain in a spruce forest ecosystem at Solling, FRG. Water Air Soil Pollut 85:1605–1611
Bredemeier M, Blanck K, Dohrenbusch A, Lamersdorf N, Meyer AC, Murach D, Parth A, Xu Y-J (1998) The Solling roof project—site characteristics, experiments and results. For Ecol Manag 101:281–293
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in the soil. Soil Biol Biochem 17:837–842
Castro A, González-Prieto SJ, Carballas T (2006) Burning effects on the distribution of organic N compounds in a 15N labelled forest soil. Geoderma 130:97–107
Corre MD, Lamersdorf NP (2004) Reversal of nitrogen saturation after long-term deposition reduction, impact on soil nitrogen cycling. Ecology 85:3090–3104
de Vries W, Posch M, Oja T, van Oene H, Kros H, Warfvinge P, Arp PA (1995) Modelling critical loads for the Solling spruce site. Ecol Model 83:283–293
Dörr N, Kaiser K, Lamersdorf N, Mikutta R, Guggenberger G (2010) Slow response of soil organic matter to the reduction in atmospheric nitrogen deposition in a Norway spruce forest. Glob Chang Biol 16:2990–3003
Emmett BA, Quarmby C (1991) The effect of harvesting intensity on the fate of applied 15N-ammonium to the organic horizons of a coniferous forest in N. Wales. Biogeochemistry 15:47–63
FAO (1998) World reference base for soil resources. World soil resources report, vol 84. FAO, Rome
Feng Z, Brumme R, Xu Y-J, Lamersdorf N (2008) Tracing the fate of mineral N compounds under high ambient N deposition in a Norway spruce forest at Solling/Germany. For Ecol Manag 255:2061–2073
Fowler D, Smith R, Muller J, Cape JN, Sutton M, Erisman JW, Fagerli H (2007) Long term trends in sulphur and nitrogen deposition in Europe and the cause of non-linearities. Water Air Soil Pollut Focus 7:41–47
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vörösmarty CJ (2004) Nitrogen cycles, past, present, and future. Biogeochemistry 70:153–226
Gundersen P, Boxman AW, Lamersdorf N, Moldan F, Andersen BR (1998) Experimental manipulation of forest ecosystems: lessons from large roof experiments. For Ecol Manag 101:339–352
Högberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Koopmans CJ, Tietema A, Boxman AW (1996) The fate of 15N enriched throughfall in two coniferous forest stands at different nitrogen deposition levels. Biogeochemistry 34:19–44
Lamersdorf N, Borken W (2004) Clean rain promotes fine root growth and soil respiration in a Norway spruce forest. Glob Chang Biol 10:1351–1362
Lipson DA, Monson RK (1998) Plant–microbe competition for soil amino acids in the alpine tundra: effects of freeze-thaw and dry-wet events. Oecologia 113:406–413
Nadelhoffer KJ, Fry B (1994) Nitrogen isotope studies in forest ecosystems. In: Lajtha K, Michener RH (eds) Stable isotopes in ecology and environmental science. Blackwell Scientific Publications, Oxford, pp 22–44
Nordin A, Schmidt IK, Shaver GR (2004) Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 85:955–962
Ryan MC, Aravena R, Gillham RW (1995) The use of 13C natural abundance to investigate the turnover of the microbial biomass and active fractions of soil organic matter under two tillage treatments. In: Lal R, Kimble J, Levine E, Stewart BA (eds) Soils and global change. CRC Press, Boca Raton, pp 351–360
Sauheitl L, Glaser B, Weigelt A (2009) Advantages of compound-specific stable isotope measurements over bulk measurements in studies on plant uptake of intact amino acids. Rapid Commun Mass Spectrom 23:3333–3342
Stange CF, Spott O, Apelt B, Russow RWB (2007) Automated and rapid online determination of 15N abundance and concentration of ammonium, nitrite or nitrate in aqueous samples by the SPINMAS technique. Isot Environ Health Stud 43:227–236
Theuerl S, Dörr N, Guggenberger G, Langer U, Kaiser K, Lamersdorf N, Buscot F (2010) Response of recalcitrant soil substances to reduced N deposition in a spruce forest soil: integrating laccase encoding genes and lignin decomposition. FEMS Microbiol Ecol 73:166–177
Tietema A, Emmett BA, Gundersen P, Kjnaas OJ, Koopmans CJ (1998) The fate of 15N-labelled nitrogen deposition in coniferous forest ecosystems. For Ecol Manag 101:19–27
Acknowledgments
We are grateful to Dirk Böttger for the management of the experimental site and his help during the sampling. We thank Pieter Wiese and Bernd Apelt for laboratory assistance and Jeannette Boguhn for EA-IRMS measurements. The work was financial supported by the German Research Foundation (PAK 12, GU 406/14-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dörr, N., Kaiser, K., Sauheitl, L. et al. Fate of ammonium 15N in a Norway spruce forest under long-term reduction in atmospheric N deposition. Biogeochemistry 107, 409–422 (2012). https://doi.org/10.1007/s10533-010-9561-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9561-z