Abstract
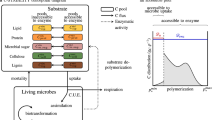
Microbiologically active biogeochemical interfaces are excellent systems to study soil functions such as pesticide degradation at the micro-scale. In particular, in the detritusphere pesticide degradation is accelerated by input of fresh organic carbon from litter into the adjacent soil. This observed priming effect suggests: (i) pesticide degradation is strongly coupled to carbon turnover, (ii) it is controlled by size and activity of the microbial community and (iii) sorption and transport of dissolved carbonaceous compounds and pesticides might regulate substrate availability and in turn decomposition processes. We present a new mechanistic 1D model (PEsticide degradation Coupled to CArbon turnover in the Detritusphere, PECCAD) which implements these hypotheses. The new model explicitly considers growth and activity of bacteria, fungi and specific pesticide degraders in response to substrate availability. Enhanced pesticide degradation due to availability of a second source of carbon (dissolved organic carbon) is implemented in the model structure via two mechanisms. First, additional substrate is utilized simultaneously with the pesticide by bacterial pesticide degraders resulting in an increase in their size and activity. Second, stimulation of fungal growth and activity by additional substrates leads directly to higher pesticide degradation via co-metabolism. Thus, PECCAD implicitly accounts for litter-stimulated production and activity of unspecific fungal enzymes responsible for co-metabolic pesticide degradation. With a global sensitivity analysis we identified high-leverage model parameters and input. In combination with appropriate experimental data, PECCAD can serve as a tool to elucidate regulation mechanisms of accelerated pesticide degradation in the detritusphere.





Similar content being viewed by others
References
Addiscott T, Smith J, Bradbury N (1995) Critical evaluation of models and their parameters. J Environ Qual 24(5):803–807
Allison SD (2012) A trait-based approach for modelling microbial litter decomposition. Ecol Lett 15(9):1058–1070. doi:10.1111/j.1461-0248.2012.01807.x
Alvarez-Cohen L, Speitel GE Jr (2001) Kinetics of aerobic cometabolism of chlorinated solvents. Biodegrad 12(2):105–126
Arnold JG, Srinivasan R, Muttiah RS, Williams JR (1998) Large area hydrologic modeling and assessment part I: model development. J Am Water Resour Assoc 34(1):73–89
Beare MH, Coleman DC, Crossley DA Jr, Hendrix PF, Odum EP (1995) A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 170(1):5–22
Beven K, Freer J (2001) Equifinality, data assimilation, and uncertainty estimation in mechanistic modelling of complex environmental systems using the GLUE methodology. J Hydrol 249(1–4):11–29. doi:10.1016/s0022-1694(01)00421-8
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fert Soils 45(2):115–131. doi:10.1007/s00374-008-0334-y
Blagodatsky SA, Richter O (1998) Microbial growth in soil and nitrogen turnover: a theoretical model considering the activity state of microorganisms. Soil Biol Biochem 30(13):1743–1755
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42(8):1275–1283. doi:10.1016/j.soilbio.2010.04.005
Blagodatsky S, Grote R, Kiese R, Werner C, Butterbach-Bahl K (2011) Modelling of microbial carbon and nitrogen turnover in soil with special emphasis on N-trace gases emission. Plant Soil 346(1):297–330. doi:10.1007/s11104-011-0821-z
Blume H-P, Brümmer GW, Horn R, Kandeler E, Kögel-Knabner I, Kretzschmar R, Stahr K, Wilke B-M (2010) Scheffer/Schachtschabel: Lehrbuch der Bodenkunde, 16th edn. Spektrum Akademischer, Heidelberg
Bosatta E, Ågren GI (1991) Dynamics of carbon and nitrogen in the organic matter of the soil: a generic theory. Am Nat 138(1):227–245
Bosatta E, Ågren GI (2003) Exact solutions to the continuous-quality equation for soil organic matter turnover. J Theor Biol 224(1):97–105. doi:10.1016/s0022-5193(03)00147-4
Braakhekke MC, Beer C, Hoosbeek MR, Reichstein M, Kruijt B, Schrumpf M, Kabat P (2011) Somprof: a vertically explicit soil organic matter model. Ecol Model 222(10):1712–1730. doi:10.1016/j.ecolmodel.2011.02.015
Brusseau ML, Sandrin SK, Li L, Yolcubal I, Jordan FL, Maier RM (2006) Biodegradation during contaminant transport in porous media: 8. The influence of microbial system variability on transport behavior and parameter determination. Water Resour Res 42 (4):W04406. doi:10.1029/2005wr004112
Carpenter SR (1981) Decay of heterogenous detritus: a general model. J Theor Biol 89(4):539–547
Cheyns K, Mertens J, Diels J, Smolders E, Springael D (2010) Monod kinetics rather than a first-order degradation model explains atrazine fate in soil mini-columns: Implications for pesticide fate modelling. Environ Pollut158 (5):1405–1411. doi:10.1016/j.envpol.2009.12.041
Criddle CS (1993) The kinetics of cometabolism. Biotechnol Bioeng 41(11):1048–1056
De Wilde T, Mertens J, Šimunek J, Sniegowksi K, Ryckeboer J, Jaeken P, Springael D, Spanoghe P (2009) Characterizing pesticide sorption and degradation in microscale biopurification systems using column displacement experiments. Environ Pollut157 (2):463–473. doi:10.1016/j.envpol.2008.09.008
Doherty J (2005) PEST: Model Independent Parameter Estimation, 5th edn. Watermark Numerical Computing, Brisbane
Dubus IG, Beulke S, Brown CD, Gottesbüren B, Dieses A (2004) Inverse modelling for estimating sorption and degradation parameters for pesticides. Pest Manag Sci 60(9):859–874. doi:10.1002/ps.893
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Chang Biol 18(6):1781–1796. doi:10.1111/j.1365-2486.2012.02665.x
Egli T (2010) How to live at very low substrate concentration. Water Res 44(17):4826–4837. doi:10.1016/j.watres.2010.07.023
Ekschmitt K, Kandeler E, Poll C, Brune A, Buscot F, Friedrich M, Gleixner G, Hartmann A, Kästner M, Marhan S, Miltner A, Scheu S, Wolters V (2008) Soil-carbon preservation through habitat constraints and biological limitations on decomposer activity. J Plant Nutr Soil Sci 171(1):27–35. doi:10.1002/jpln.200700051
Estrella MR, Brusseau ML, Maier RS, Pepper IL, Wierenga PJ, Miller RM (1993) Biodegradation, sorption, and transport of 2,4-dichlorophenoxyacetic acid in saturated and unsaturated soils. Appl Environ Microbiol 59(12):4266–4273
Fan Z, Neff JC, Wickland KP (2010) Modeling the production, decomposition, and transport of dissolved organic carbon in boreal soils. Soil Sci 175(5):223–232. doi:10.1097/SS.0b013e3181e0559a
Feng Y (2009) K-model-A continuous model of soil organic carbon dynamics: theory. Soil Sci 174(9):482–493. doi:10.1097/SS.0b013e3181bb0f80
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecol 88(6):1354–1364. doi:10.1890/05-1839
Fontaine S, Barot S (2005) Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol Lett 8(10):1075–1087. doi:10.1111/j.1461-0248.2005.00813.x
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35(6):837–843. doi:10.1016/s0038-0717(03)00123-8
Gaillard V, Chenu C, Recous S (2003) Carbon mineralisation in soil adjacent to plant residues of contrasting biochemical quality. Soil Biol Biochem 35(1):93–99
Garnier P, Néel C, Mary B, Lafolie F (2001) Evaluation of a nitrogen transport and transformation model in a bare soil. Eur J Soil Sci 52(2):253–268
Gaultier J, Farenhorst A, Cathcart J, Goddard T (2008) Degradation of [carboxyl-14C] 2,4-D and [ring-U-14C] 2,4-D in 114 agricultural soils as affected by soil organic carbon content. Soil Biol Biochem 40(1):217–227. doi:10.1016/j.soilbio.2007.08.003
Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176(1):20–30. doi:10.1016/j.plantsci.2008.09.014
Ghafoor A, Jarvis NJ, Thierfelder T, Stenström J (2011) Measurements and modeling of pesticide persistence in soil at the catchment scale. Sci Total Environ 409(10):1900–1908. doi:10.1016/j.scitotenv.2011.01.049
Ghani A, Wardle DA (2001) Fate of 14C from glucose and the herbicide metsulfuron-methyl in a plant-soil microcosm system. Soil Biol Biochem 33(6):777–785. doi:10.1016/s0038-0717(00)00225-x
Gignoux J, House J, Hall D, Masse D, Nacro HB, Abbadie L (2001) Design and test of a generic cohort model of soil organic matter decomposition: the SOMKO model. Glob Ecol and Biogeogr 10(6):639–660
Gjettermann B, Styczen M, Hansen HCB, Vinther FP, Hansen S (2008) Challenges in modelling dissolved organic matter dynamics in agricultural soil using DAISY. Soil Biol Biochem 40(6):1506–1518. doi:10.1016/j.soilbio.2008.01.005
Gonod LV, Martin-Laurent F, Chenu C (2006) 2,4-D impact on bacterial communities, and the activity and genetic potential of 2,4-D degrading communities in soil. FEMS Microbiol Ecol 58(3):529–537. doi:10.1111/j.1574-6941.2006.00159.x
Harder W, Dijkhuizen L (1982) Strategies of mixed substrate utilization in microorganisms. Philos Trans R Soc Lond Ser B: Biol Sci 297(1088):459–480
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105(12):1422–1432
Henriksen TM, Breland TA (1999) Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol Biochem 31(8):1121–1134. doi:10.1016/s0038-0717(99)00030-9
Higuchi R, Fockler C, Dollinger G, Watson R (1993) Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Nat Biotechnol 11(9):1026–1030
Ingwersen J, Poll C, Streck T, Kandeler E (2008) Micro-scale modelling of carbon turnover driven by microbial succession at a biogeochemical interface. Soil Biol Biochem 40(4):872–886
Jenkinson DS, Rayner JH (1977) The turnover of soil organic matter in some of the Rothamsted classical experiments. Soil Sci 123(5):298–305
Jennings DH, Lysek G (1999) Fungal biology: understanding the fungal lifestyle, 2nd edn. Bios Scientific Publishers, Oxford
Jensen PH, Hansen HCB, Rasmussen J, Jacobsen OS (2004) Sorption-controlled degradation kinetics of MCPA in soil. Environ Sci Technol 38(24):6662–6668
Kandeler E, Luxhøi J, Tscherko D, Magid J (1999) Xylanase, invertase and protease at the soil-litter interface of a loamy sand. Soil Biol Biochem 31(8):1171–1179
Kögel-Knabner I (2002) The macromolecular organic composition of Plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34(2):139–162
Köhne JM, Köhne S, Šimunek J (2006) Multi-process herbicide transport in structured soil columns: experiments and model analysis. J Contaminant Hydrology 85(1–2):1–32. doi:10.1016/j.jconhyd.2006.01.001
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42(9):1363–1371. doi:10.1016/j.soilbio.2010.04.003
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32(11–12):1485–1498. doi:10.1016/s0038-0717(00)00084-5
Langner HW, Inskeep WP, Gaber HM, Jones WL, Das BS, Wraith JM (1998) Pore water velocity and residence time effects on the degradation 2,4-D during transport. Environ Sci Technol 32(9):1308–1315. doi:10.1021/es970834q
Leistra M, van der Linden AMA, Boesten JJTI, Tiktak A, van den Berg F (2001) PEARL model for pesticide behaviour and emissions in soil-plant systems: Description of the processes in FOCUS PEARL version 1.1.1. Alterra Rep. 013. Alterra, Wageningen
Lendenmann U, Egli T (1998) Kinetic models for the growth of Escherichia coil with mixtures of sugars under carbon-limited conditions. Biotechnol Bioeng 59(1):99–107
Lerch TZ, Dignac MF, Nunan N, Bardoux G, Barriuso E, Mariotti A (2009) Dynamics of soil microbial populations involved in 2,4-D biodegradation revealed by FAME-based Stable Isotope Probing. Soil Biol Biochem 41(1):77–85
Macey R, Oster G, Zahnley T (2000) Berkeley Madonna User’s Guide 8.0. University of California, Department of Molecular and Cellular Biology, Berkeley
Manzoni S, Porporato A (2009) Soil carbon and nitrogen mineralization: theory and models across scales. Soil Biol Biochem 41(7):1355–1379
Marschner B, Kalbitz K (2003) Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 113(3–4):211–235. doi:10.1016/s0016-7061(02)00362-2
McGuire KL, Treseder KK (2010) Microbial communities and their relevance for ecosystem models: decomposition as a case study. Soil Biol Biochem 42(4):529–535. doi:10.1016/j.soilbio.2009.11.016
McKay MD, Beckman RJ, Conover WJ (1979) Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 21(2):239–245
Mertens J, Kahl G, Gottesbüren B, Vanderborght J (2009) Inverse modeling of pesticide leaching in lysimeters: local versus global and sequential single-objective versus multiobjective approaches. Vadose Zone J 8(3):793–804. doi:10.2136/vzj2008.0029
Michalzik B, Tipping E, Mulder J, Gallardo Lancho JF, Matzner E, Bryant CL, Clarke N, Lofts S, Vicente Esteban MA (2003) Modelling the production and transport of dissolved organic carbon in forest soils. Biogeochem 66(3):241–264. doi:10.1023/b:biog.0000005329.68861.27
Millington RJ, Quirk JP (1961) Permeability of porous solids. Trans Faraday Soc 57:1200–1207. doi:10.1039/tf9615701200
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76(2):151–174
Neff JC, Asner GP (2001) Dissolved organic carbon in terrestrial ecosystems: synthesis and a model. Ecosyst 4(1):29–48. doi:10.1007/s100210000058
Neill C, Gignoux J (2006) Soil organic matter decomposition driven by microbial growth: a simple model for a complex network of interactions. Soil Biol Biochem 38(4):803–811
Niklaus PA, Falloon P (2006) Estimating soil carbon sequestration under elevated CO2 by combining carbon isotope labelling with soil carbon cycle modelling. Glob Change Biol 12(10):1909–1921. doi:10.1111/j.1365-2486.2006.01215.x
Nowak KM, Miltner A, Gehre M, Schäffer A, Kästner M (2011) Formation and fate of bound residues from microbial biomass during 2, 4-D degradation in soil. Environ Sci Technol 45(3):999–1006. doi:10.1021/es103097f
Panikov NS (1995) Microbial growth kinetics, 1st edn. Chapman & Hall, Weinheim
Panikov NS (1999) Understanding and prediction of soil microbial community dynamics under global change. Appl Soil Ecol 11(2–3):161–176. doi:10.1016/s0929-1393(98)00143-7
Pansu M, Martineau Y, Saugier B (2009) A modelling method to quantify in situ the input of carbon from roots and the resulting C turnover in soil. Plant Soil 317(1–2):103–120. doi:10.1007/s11104-008-9791-1
Parnas H (1976) A theoretical explanation of the priming effect based on microbial growth with two limiting substrates. Soil Biol Biochem 8(2):139–144
Parton WJ (1993) Observations and modeling of biomass and soil organic matter dynamics for the grassland biome worldwide. Glob Biogeochem Cycle 7(4):785–809
Paul EA (2007) Soil microbiology, ecology, and biochemistry, 3rd edn. Academic Press, Amsterdam
Paustian K, Schnürer J (1987) Fungal growth response to carbon and nitrogen limitation: a theoretical model. Soil Biol Biochem 19(5):613–620
PDE Solutions Inc. (2011) FlexPDE 6.20 - finite element model builder for Partial Differential Equations. WA, USA
Poll C, Ingwersen J, Stemmer M, Gerzabek MH, Kandeler E (2006) Mechanisms of solute transport affect small-scale abundance and function of soil microorganisms in the detritusphere. Eur J Soil Sci 57(4):583–595
Poll C, Marhan S, Ingwersen J, Kandeler E (2008) Dynamics of litter carbon turnover and microbial abundance in a rye detritusphere. Soil Biol Biochem 40(6):1306–1321
Poll C, Pagel H, Devers-Lamrani M, Martin-Laurent F, Ingwersen J, Streck T, Kandeler E (2010) Regulation of bacterial and fungal MCPA degradation at the soil-litter interface. Soil Biol Biochem 42(10):1879–1887. doi:10.1016/j.soilbio.2010.07.013
Richter O, Diekkrüger B, Nörtersheuser P (1996) Environmental fate modelling of pesticides: from the laboratory to the field scale. VCH, Weinheim
Roulier S, Jarvis N (2003) Modeling macropore flow effects on pesticide leaching: inverse parameter estimation using microlysimeters. J Environ Qual 32(6):2341–2353
Rovira P, Rovira R (2010) Fitting litter decomposition datasets to mathematical curves: towards a generalised exponential approach. Geoderma 155(3–4):329–343. doi:10.1016/j.geoderma.2009.11.033
Russell JB, Cook GM (1995) Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev 59(1):48–62
Saltelli A (2008) Global sensitivity analysis. the primer. Wiley, Chichester
Scharnagl B, Vrugt JA, Vereecken H, Herbst M (2010) Information content of incubation experiments for inverse estimation of pools in the Rothamsted carbon model: a Bayesian perspective. Biogeosci 7(2):763–776
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35(4):549–563. doi:10.1016/s0038-0717(03)00015-4
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478(7367):49–56. doi:10.1038/nature10386
Scow KM, Johnson CR (1996) Effect of Sorption on Biodegradation of Soil Pollutants. Adv Agron 58 (C):1–56. doi:10.1016/s0065-2113(08)60252-7
Shaw LJ, Burns RG (2003) Biodegradation of Organic Pollutants in the Rhizosphere. Adv Appl Microbiol 53–60:1. doi:10.1016/s0065-2164(03)53001-5
Shelton DR, Doherty MA (1997) A model describing pesticide bioavailability and biodegradation in soil. Soil Sci Soc Am J 61(4):1078–1084
Simkins S, Alexander M (1984) Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol 47(6):1299–1306
Šimůnek J, Šejna M, Saito H, Sakai M, van Genuchten MT (2008) The HYDRUS-1D Software Package for Simulating the Movement of Water, Heat, and Multiple Solutes in Variably Saturated Media, Version 4.08. HYDRUS Software Series 3. Department of Environmental Sciences, University of California Riverside, Riverside
Skjemstad JO, Spouncer LR, Cowie B, Swift RS (2004) Calibration of the Rothamsted organic carbon turnover model (RothC ver. 26.3), using measurable soil organic carbon pools. Aust J Soil Res 42 (1):79–88. doi:10.1071/sr03013
Smith P, Smith JU, Powlson DS, McGill WB, Arah JRM, Chertov OG, Coleman K, Franko U, Frolking S, Jenkinson DS, Jensen LS, Kelly RH, Klein-Gunnewiek H, Komarov AS, Li C, Molina JAE, Mueller T, Parton WJ, Thornley JHM, Whitmore AP (1997) A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 81(1–2):153–225. doi:10.1016/s0016-7061(97)00087-6
Streck T, Richter J (1999) Field-scale study of chlortoluron movement in a sandy soil over winter: II. Modeling. J Environ Qual 28(6):1824–1831
Todd-Brown KEO, Hopkins FM, Kivlin SN, Talbot JM, Allison SD (2012) A framework for representing microbial decomposition in coupled climate models. Biogeochem 109(1–3):19–33. doi:10.1007/s10533-011-9635-6
Totsche KU, Rennert T, Gerzabek MH, Kögel-Knabner I, Smalla K, Spiteller M, Vogel HJ (2010) Biogeochemical interfaces in soil: the interdisciplinary challenge for soil science. J Plant Nutr Soil Sci 173(1):88–99. doi:10.1002/jpln.200900105
van Griensven A, Meixner T, Grunwald S, Bishop T, Diluzio M, Srinivasan R (2006) A global sensitivity analysis tool for the parameters of multi-variable catchment models. J Hydrol 324(1–4):10–23
Van Veen JA, Paul EA (1981) Organic carbon dynamics in grassland soils. 1. Background information and computer simulation. Can J Soil Sci 61(2):185–201. doi:10.4141/cjss81-024
Vrugt JA, Robinson BA (2007) Improved evolutionary optimization from genetically adaptive multimethod search. Proc Natl Acad Sci U S A 104(3):708–711. doi:10.1073/pnas.0610471104
Wallenstein MD, Hall EK (2012) A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochem 109(1–3):35–47. doi:10.1007/s10533-011-9641-8
Wallenstein M, Stromberger M, Bell C (2012) Bridging the gap between modelers and experimentalists. Eos Trans AGU 93 (32). doi:10.1029/2012eo320005
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A 95(12):6578–6583. doi:10.1073/pnas.95.12.6578
WRB (2006) World reference base for soil resources 2006. World Soil Res Rep, FAO
Wutzler T, Reichstein M (2008) Colimitation of decomposition by substrate and decomposers: a comparison of model formulations. Biogeosci 5(3):749–759
Young IM, Crawford JW, Nunan N, Otten W, Spiers A (2009) Chapter 4 Microbial Distribution in Soils. Physics and Scaling. Adv Agron 100 (C):81–121. doi:10.1016/s0065-2113(08)00604-4
Yurova A, Sirin A, Buffam I, Bishop K, Laudon H (2008) Modeling the dissolved organic carbon output from a boreal mire using the convection-dispersion equation: importance of representing sorption. Water Resour Res 44(7):W07411. doi:10.1029/2007wr006523
Zander C, Streck T, Kumke T, Altfelder S, Richter J (1999) Field-scale study of chlortoluron movement in a sandy soil over winter: I. Experiments. J Environ Qual 28(6):1817–1823
Zimmermann M, Leifeld J, Schmidt MWI, Smith P, Fuhrer J (2007) Measured soil organic matter fractions can be related to pools in the RothC model. Eur J Soil Sci 58(3):658–667. doi:10.1111/j.1365-2389.2006.00855.x
Acknowledgments
We thank Kathleen Regan for English corrections and gratefully acknowledge the funding (KA 1590/5-2 and STR 481/3-2) by Deutsche Forschungsgemeinschaft (DFG) in the frame of the priority program SPP 1315: “Biogeochemical Interfaces in Soil”. We gratefully appreciate the valuable recommendations of three unknown reviewers.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pagel, H., Ingwersen, J., Poll, C. et al. Micro-scale modeling of pesticide degradation coupled to carbon turnover in the detritusphere: model description and sensitivity analysis. Biogeochemistry 117, 185–204 (2014). https://doi.org/10.1007/s10533-013-9851-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9851-3