Abstract
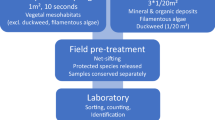
Littoral macroinvertebrates are increasingly used for assessing the ecological status of lakes according to the EU Water Framework Directive. This requires harmonised sampling methods, but information on the appropriate spatial scale of the sampling as well as on the adequate sample sizes are mostly lacking. In this study, we compared the spatial variability of littoral (<1.2 m water depth) macroinvertebrate community composition within habitats and within sites to test whether habitat-specific sampling can reduce their spatial variability. Furthermore, we determined the sample size necessary to obtain maximum species richness for a given habitat type. Spatial variability of macroinvertebrate community composition was significantly lower within habitats than within sampling sites, except for communities of coarse woody debris. Species–area curves revealed that a sample size of 1 m2 per habitat was not sufficient to obtain the maximum species richness due to the dominance of rare species, which suggests that compilation of taxon inventories may require more exhaustive sampling with sampling sizes substantially larger than 1 m2. Separate analysis for species assigned to incidence classes showed that a mean area of 0.63 m2 per habitat is sufficient to record all species with frequent and medium incidences, and 76% of the rare species. We conclude that habitat-specific sampling is an effective way to reduce the inherent spatial variability of littoral macroinvertebrate communities and that a sample size of 0.63 m2 per habitat is sufficient to represent their dominant and subdominant elements. The application of this adequate sample size to other lake types than large oligotrophic lakes has to be exercised with caution, in particular if community composition and richness patterns differ. However, our results are based on data from lakes that represent the typical lake type found throughout the Central Baltic ecoregion ensuring its wider applicability in this ecoregion.


Similar content being viewed by others
References
Anderson, M. J., 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62: 245–253.
Bloesch, J., 1995. Mechanisms, measurement and importance of sediment resuspension in lakes. Marine and Freshwater Research 46: 295–304.
Brauns, M., X.-F. Garcia, M. T. Pusch & N. Walz, 2007a. Eulittoral macroinvertebrate communities of lowland lakes: discrimination among trophic states. Freshwater Biology 52: 1022–1032.
Brauns, M., X.-F. Garcia, N. Walz & M. T. Pusch, 2007b. Effects of human shoreline development on littoral macroinvertebrates in lowland lakes. Journal of Applied Ecology 44: 1138–1144.
Brauns, M., X.-F. Garcia & M. T. Pusch, 2008. Potential effects of water-level fluctuations on littoral invertebrates in lowland lakes. Hydrobiologia 613: 5–12.
Bray, J. R. & J. T. Curtis, 1957. Ordination of the upland forest communities of southwestern Wisconsin. Ecological Monographs 27: 325–349.
Cao, Y., D. Williams & N. E. Williams, 1998. How important are rare species in aquatic community ecology and bioassessment? Limnology and Oceanography 43: 1403–1409.
Cao, Y., D. P. Larsen & R. S.-J. Thorne, 2001. Rare species in multivariate analysis for bioassessment: some considerations. Journal of the North American Benthological Society 20: 144–153.
Chao, A., R. K. Colwell, C. W. Lin & N. J. Gotelli, 2009. Sufficient sampling for asymptotic species richness estimators. Ecology 90: 1125–1133.
Cheruvelil, C. S., P. A. Soranno & R. D. Serbin, 2000. Macroinvertebrates associated with submerged macrophytes: sample size and power to detect effects. Hydrobiologia 441: 133–139.
Cucherousset, J., F. Santoul, J. Figuerola & R. Cereghino, 2008. How do biodiversity patterns of river animals emerge from the distributions of common and rare species? Biological Conservation 141: 2984–2992.
Cyr, H., 1998. Effects of wave disturbance and substrate slope on sediment characteristics in the littoral zone of small lakes. Canadian Journal of Fisheries and Aquatic Sciences 55: 967–976.
Czachorowski, S., 1993. Vertical distribution of caddis larvae in the northeastern Polish lakes. Braueria 20: 7–9.
Dall, P. C., C. Lindegaard & P. M. Jonasson, 1990. In lake variations in the composition of zoobenthos in the littoral zone of lake Esrom. Verhandlungen der Internationalen Vereinigung für Limnologie 24: 613–620.
Death, R. G., 1995. Spatial patterns in benthic invertebrate community structure – products of habitat stability or are they habitat specific. Freshwater Biology 33: 455–467.
Donohue, I., L. A. Donohue, B. N. Ainin & K. Irvine, 2009a. Assessment of eutrophication pressure on lakes using littoral invertebrates. Hydrobiologia 633: 105–122.
Donohue, I., A. L. Jackson, M. T. Pusch & K. Irvine, 2009b. Nutrient enrichment homogenizes lake benthic assemblages at local and regional scales. Ecology 90: 3470–3477.
Downes, B. J., P. S. Lake & E. S. G. Schreiber, 1993. Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization. Freshwater Biology 30: 119–132.
European Commission, 2000. Council Directive 2000/60/EC establishing a framework for community action in the field of water policy. Official Journal No. L 321/1: 1-72.
Garcia-Criado, F., E. Becares, C. Fernandez-Alaez & M. Fernandez-Alaez, 2005. Plant-associated invertebrates and ecological quality in some Mediterranean shallow lakes: implications for the application of the EC Water Framework Directive. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 31–50.
Harrison, S. S. C. & A. G. Hildrew, 2001. Epilithic communities and habitat heterogeneity in a lake littoral. Journal of Animal Ecology 70: 692–707.
Hellawell, J. M., 1986. Biological indicators of freshwater pollution and environmental management. Elsevier Applied Science Publishers, London.
Hofmann, H., A. Lorke & F. Peeters, 2008. The relative importance of wind and ship waves in the littoral zone of a large lake. Limnology and Oceanography 53: 368–380.
Irvine, K., 2004. Classifying ecological status under the European Water Framework Directive: the need for monitoring to account for natural variability. Aquatic Conservation: Marine and Freshwater Ecosystems 14: 107–112.
James, M. R., M. A. Weatherhead, C. Stanger & E. Graynoth, 1998. Macroinvertebrate distribution in the littoral zone of Lake Coleridge, South island, New Zealand – effects of habitat stability, wind exposure, and macrophytes. New Zealand Journal of Marine and Freshwater Research 32: 287–305.
Nijboerg, R. C. & P. F. M. Verdonschot, 2004. Rare and common macroinvertebrates: definition of distribution classes and their boundaries. Archiv für Hydrobiologie 161: 45–64.
Raunio, J. & M. Anttila-Huhtinen, 2008. Sample size determination for soft-bottom sampling in large rivers and comparison with the Chironomid Pupal Exuvial Technique (CPET). River Research and Application 24: 835–843.
Rowan, D. J., J. Kalff & J. B. Rasmussen, 1992. Estimating the mud deposition boundary depth in lakes from wave theory. Canadian Journal of Fisheries and Aquatic Sciences 49: 2490–2497.
Särkkä, J., 1983. A quantitative ecological investigation of the littoral zoobenthos of an oligotrophic Finnish lake. Annales Zoologici Fennici 20: 157–178.
Schindler, D. E. & M. D. Scheuerell, 2002. Habitat coupling in lake ecosystems. Oikos 98: 177–189.
Solheim, A. L., S. Rekolainen, S. J. Moe, L. Carvalho, G. Phillips, R. Ptacnik, W. E. Penning, L. G. Toth, C. O’Toole, A. K. L. Schartau & T. Hesthagen, 2008. Ecological threshold responses in European lakes and their applicability for the Water Framework Directive (WFD) implementation: synthesis of lakes results from the REBECCA project. Aquatic Ecology 42: 317–334.
Solimini, A. G., G. Free, I. Donohue, K. Irvine, M. T. Pusch, B. Rossaro, L. Sandin & A. C. Cardoso, 2006. Using Benthic Macroinvertebrates to Assess Ecological Status of Lakes – Current Knowledge and Way Forward to Support WFD Implementation. Institute for Environment and Sustainability, Ispra 1-47. http://ies.jrc.ec.europa.eu/uploads/fileadmin/Documentation/Reports/RWER/EUR_2006-2007/EUR_22347_EN_01.pdf.
Stendera, S. & R. K. Johnson, 2008. Habitat-specific stability and persistence of benthic invertebrate communities in boreal lakes. Fundamental and Applied Limnology 171: 311–322.
Stoffels, R. J., K. R. Clarke & G. Closs, 2005. Spatial scale and benthic community organisation in the littoral zones of large oligotrophic lakes: potential for cross-scale interactions. Freshwater Biology 50: 1131–1145.
Tolonen, K. T. & H. Hämäläinen, 2010. Comparison of sampling methods and habitat types for detecting impacts on lake littoral macroinvertebrate assemblages along a gradient of human disturbance. Fundamental and Applied Limnology 176: 43–59.
Tolonen, K. T., H. Hämäläinen, I. J. Holopainen & J. Karjalainen, 2001. Influences of habitat type and environmental variables on littoral macroinvertebrate communities in a large lake system. Archiv für Hydrobiologie 152: 39–67.
Veijola, H., J. J. Meriläinen & V. Marttila, 1996. Sample size on the monitoring of benthic macrofauna in the profundal of lakes: evaluation of the precision of estimates. Hydrobiologia 322: 301–315.
Vlek, H. E., F. Sporka & I. Krno, 2006. Influence of macroinvertebrate sample size on bioassessment of streams. Hydrobiologia 566: 523–542.
Weatherhead, M. A. & M. R. James, 2001. Distribution of macroinvertebrates in relation to physical and biological variables in the littoral zone of nine New Zealand lakes. Hydrobiologia 462: 115–129.
Acknowledgements
We thank Attila Öztürk, Daniel (DON) Graeber, Judith Reise and Katrin Quiel for their assistance in the field and in the laboratory. Comments from Friederike Gabel, Gwendolin Porst and Martin Pusch substantially improved earlier versions of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: B. Oertli
Rights and permissions
About this article
Cite this article
Schreiber, J., Brauns, M. How much is enough? Adequate sample size for littoral macroinvertebrates in lowland lakes. Hydrobiologia 649, 365–373 (2010). https://doi.org/10.1007/s10750-010-0284-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0284-x