Abstract
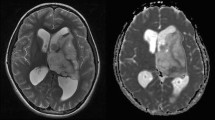
Bevacizumab is an anti-vascular endothelial growth factor (VEGF) antibody with activity against recurrent malignant glioma inducing high rates of objective responses as assessed by magnetic resonance imaging (MRI). However, the mechanisms of the anti-tumor action of bevacizumab are controversial. In particular, it is unclear whether and when bevacizumab induces hypoxia in gliomas. Vascular normalization with hyperperfusion and enhanced oxygen delivery to the tumor has been suggested as an alternative mechanism. We analyzed diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps in 18 consecutive patients with recurrent malignant glioma before and after exposure to bevacizumab. Stroke-like lesions with diffusion restriction on DWI and corresponding ADC decrease were induced by bevacizumab within the previously enhancing tumor area in 13 of 18 patients. These lesions were detectable as early as 4 weeks after initiation of therapy and were maintained for up to 80 weeks. In one patient, an ADC-decreased lesion was biopsied, and histology showed atypical necrosis and nuclear hypoxia-inducible factor 1alpha upregulation but no tumor recurrence. Normalized regional cerebral blood flow (rCBF) and regional cerebral blood volume (rCBV) were analyzed in selected patients. Both parameters were decreased in responders with diffusion-restricted lesions. Within the tumor bed, bevacizumab induces diffusion-restricted lesions in the presence of reduced rCBF and rCBV. The cause of these alterations is unclear but may involve atypical necrosis and chronic hypoxia.



Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CR:
-
Complete response
- DWI:
-
Diffusion-weighted imaging
- HIF-1α:
-
Hypoxia-inducible factor-1α
- MR:
-
Minor response
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- rCBF:
-
Regional cerebral blood flow
- rCBV:
-
Regional cerebral blood volume
- SD:
-
Stable disease
- VEGF:
-
Vascular endothelial growth factor
References
Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 27:4733–4740
Sathornsumetee S, Cao Y, Marcello JE, Herndon JE, McLendon RE, Desjardins A, Friedman HS, Dewhirst MW, Vredenburgh JJ, Rich JN (2008) Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol 26:271–278
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Batchelor TT, Sorensen AG, di Tomaso E, Zhang W, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen P, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Macdonald DR, Cascino TL, Schold SCJ, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Server A, Kulle B, Maehlen J, Josefsen R, Schellhorn T, Kumar T, Langberg CW, Nakstad PH (2009) Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema. Acta Radiol 50:682–689
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Castillo M, Smith JK, Kwock L, Wilber K (2001) Apparent diffusion coefficients in the evaluation of high-grade cerebral gliomas. ANJR Am J Neuroradiol 22:60–64
Pope WB, Kim HJ, Huo J, Alger J, Brown S, Gjertson D, Sai V, Young JR, Tekchandani L, Cloughesy T, Mischel PS, Lai A, Nghiemphu P, Rahmanuddin S, Goldin J (2009) Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252:182–189
Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, McKeever PE, Chenevert TL (2006) Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24:1131–1142
Fischer I, Cunliffe CH, Bollo RJ, Raza S, Monoky D, Chiriboga L, Parker EC, Golfinos JG, Kelly PJ, Knopp EA, Gruber ML, Zagzag D, Narayana A (2008) High-grade glioma before and after treatment with radiation and Avastin: initial observations. Neurooncology 10:700–708
Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, Batchelor TT, di Tomaso E, Duda DG, Munn LL, Fukumura D, Sorensen AG, Jain RK (2009) Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol 27:2542–2552
Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes H, Menger MD, Ullrich A, Vajkoczy P (2004) Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 18:338–340
Ronellenfitsch MW, Brucker DP, Burger MC, Wolking S, Tritschler F, Rieger J, Wick W, Weller M, Steinbach JP (2009) Antagonism of the mammalian target of rapamycin selectively mediates metabolic effects of epidermal growth factor receptor inhibition and protects human malignant glioma cells from hypoxia-induced cell death. Brain 132:1509–1522
Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM (2003) Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3:347–361
Zheng X, Jiang F, Katakowski M, Kalkanis SN, Hong X, Zhang X, Zhang ZG, Yang H, Chopp M (2007) Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci 98:674–684
Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15:220–231
Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, Park KR, Ferrara N, Jain RK, Suit HD, Boucher Y (2000) Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 60:5565–5570
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn JJ, Jain RK (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553–563
Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M, Blaschke B, Meyermann R, Reifenberger G, Weller M, Wick W (2007) Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 25:3357–3361
Acknowledgments
The Dr. Senckenberg Institute of Neurooncology is supported by the Dr. Senckenberg Foundation and the Hertie Foundation. J.S. is “Hertie Professor of Neurooncology”.
Disclosures
Prof. Steinbach has served as a consultant for Roche, the European distributor of bevacizumab (Avastin). The other authors report no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rieger, J., Bähr, O., Müller, K. et al. Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J Neurooncol 99, 49–56 (2010). https://doi.org/10.1007/s11060-009-0098-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-0098-8