Abstract
Purpose
To establish an in vitro-in vivo level A correlation (IVIVC) for pramipexole slow-release formulations.
Methods
The IVIVC was developed based on data from an immediate-release (IR) and three slow-release (SR) formulations of pramipexole; a fourth SR formulation was used for validation purposes. In vitro dissolution profiles were obtained from all SR formulations. Fifteen volunteers received all pramipexole formulations in a randomized cross-over trial. Data were analyzed using the population modelling approach as implemented in NONMEM VI.
Results
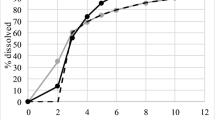
Dissolution profiles of the SR formulations were described by the Weibull model. The pharmacokinetics of the IR formulation were described by a two-compartment disposition model with first-order absorption. Difference between the in vivo and in vitro estimates of the release rate constants (kd) from the Weibull function suggests the release process occurs faster in vivo. Pharmacokinetic profiles for SR formulations were described based on the in vitro release model with kd increased in 0.058 h−1 and the population pharmacokinetic model developed from the IR formulation.
Conclusion
A level A IVIVC was established and evaluated for the pramipexole SR formulations, which can be used in the future as a surrogate to avoid certain bioequivalence studies.







Similar content being viewed by others
References
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76.
Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, et al. European federation of neurological societies, and movement disorder society-European section. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson’s disease. Eur J Neurol. 2006;13:1186–202.
Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, et al. European Federation of Neurological Societies, and Movement Disorder Society-European Section. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies and the Movement Disorder Society-European Section. Part I: early (uncomplicated) Parkinson’s disease. Eur J Neurol. 2006;13:1170–85.
Schapira AH. Present and future drug treatment for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1472–8.
Ryan M, Slevin JT. Restless legs syndrome. Am J Health Syst Pharm. 2006;63:1599–612.
Dutta S, Qiu Y, Samara E, Cao G, Granneman GR. Once-a-day extended-release dosage form of divalproex sodium III: development and validation of a Level A in vitro-in vivo correlation (IVIVC). J Pharm Sci. 2005;94:1949–56.
Food and Drug Administration. Guidance for industry extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070239.pdf (accessed Nov/25/2009).
The European Agency for the Evaluation of Medicinal Products. Note for guidance on quality of modified release products: a.oral dosage forms B.Transdermal Dosage Forms Section I (Quality). http://www.emea.europa.eu/pdfs/human/qwp/060496en.pdf (accessed November/25/2009).
Uppoor VR. Regulatory perspectives on in vitro (dissolution)/in vivo (bioavailability) correlations. J Control Release. 2001;72:127–32.
Liu Y, Schwartz JB, Schnaare RL, Sugita ET. A multi-mechanistic drug release approach in a bead dosage form and in vitro/in vivo correlations. Pharm Dev Technol. 2003;8:409–17.
Modi NB, Lam A, Lindemulder E, Wang B, Gupta SK. Application of in vitro-in vivo correlations (IVIVC) in setting formulation release specifications. Biopharm Drug Dispos. 2000;21:321–6.
Souliman S, Blanquet S, Beyssac E, Cardot JM. A level A in vitro/in vivo correlation in fasted and fed states using different methods: applied to solid immediate release oral dosage form. Eur J Pharm Sci. 2006;27:72–9.
Sunesen VH, Pedersen BL, Kristensen HG, Mullertz A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur J Pharm Sci. 2005;24:305–13.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
Beal SL, Sheiner LB. NONMEM Users Guides, Icon Development Solutions, Ellicot City, Maryland, USA, 1989–2006.
Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994;22:431–45.
Karlsson M, Holford NH. A tutorial on visual predictive checks. www.page-meeting.org/?abstract=1434.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82:17–20.
Rossenu S, Gaynor C, Vermeulen A, Cleton A, Dunne A. A nonlinear mixed effects IVIVC model for multi-release drug delivery systems. J Pharmacokinet Pharmacodyn. 2008;35:423–41.
O’Hara T, Hayes S, Davis J, Devane J, Smart T, Dunne A. In vivo-in vitro correlation (IVIVC) modeling incorporating a convolution step. J Pharmacokinet Pharmacodyn. 2001;28:277–98.
Buchwald P. Direct, differential-equation-based in-vitro-in-vivo correlation (IVIVC) method. J Pharm Pharmacol. 2003;55:495–504.
Adams E, Coomans D, Smeyers-Verbeke J, Massart DL. Non-linear mixed effects models for the evaluation of dissolution profiles. Int J Pharm. 2002;240:37–53.
Bonferoni MC, Rossi S, Ferrari F, Bertoni M, Bolhuis GK, Caramella C. On the employment of lambda carrageenan in a matrix system. III. Optimization of a lambda carrageenan-HPMC hydrophilic matrix. J Control Release. 1998;51:231–9.
Koester LS, Ortega GG, Mayorga P, Bassani VL. Mathematical evaluation of in vitro release profiles of hydroxypropylmethylcellulose matrix tablets containing carbamazepine associated to beta-cyclodextrin. Eur J Pharm Biopharm. 2004;58:177–9.
Varma MV, Kaushal AM, Garg S. Influence of micro-environmental pH on the gel layer behavior and release of a basic drug from various hydrophilic matrices. J Control Release. 2005;103:499–510.
Bressolle F, Gomeni R, Alric R, Royer-Morrot MJ, Necciari J. A double Weibull input function describes the complex absorption of sustained-release oral sodium valproate. J Pharm Sci. 1994;83:1461–4.
Rietbrock S, Merz PG, Fuhr U, Harder S, Marschner JP, Loew D, et al. Absorption behavior of sulpiride described using Weibull functions. Int J Clin Pharmacol Ther. 1995;33:299–303.
Zhou H. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol. 2003;43:211–27.
Kvernmo T, Hartter S, Burger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther. 2006;28:1065–78.
Pinter MM, Pogarell O, Oertel WH. Efficacy, safety, and tolerance of the non-ergoline dopamine agonist pramipexole in the treatment of advanced Parkinson’s disease: a double blind, placebo controlled, randomised, multicentre study. J Neurol Neurosurg Psychiatry. 1999;66:436–41.
Balan G, Timmins P, Greene DS, Marathe PH. In-vitro in-vivo correlation models for glibenclamide after administration of metformin/glibenclamide tablets to healthy human volunteers. J Pharm Pharmacol. 2000;52:831–8.
Food and Drug Administration. Guidance for industry: SUPAC-MR: modified release solid oral dosage forms; scale-up and post-approval changes: chemistry, manufacturing and controls, in vitro dissolution testing, and in vivo bioequivalence documentation. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070640.pdf (accessed November/25/2009).
Acknowledgements
The authors acknowledge Silke Luedtke and Jochen Scher of the Departments of Bioanalytics and Analytics, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soto, E., Haertter, S., Koenen-Bergmann, M. et al. Population In Vitro-In Vivo Correlation Model for Pramipexole Slow-Release Oral Formulations. Pharm Res 27, 340–349 (2010). https://doi.org/10.1007/s11095-009-0027-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-0027-8