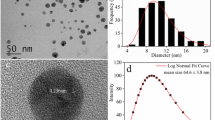
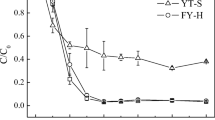
Abstract
Engineered nanoparticles (NP) like Ag and TiO2 offer unique properties for various applications. Thus, the entry of the NP in soil environments is expected to increase in the future due to their growing industrial use. To avoid potential hazards due to these anthropogenic products, NP behavior in the environment should be well understood. In natural soil solutions, we investigated NOM adsorption onto Ag and TiO2 NP and its influence on NP colloidal stability. Therefore, we extracted soil solutions from a floodplain soil (Fluvisol) and a farmland soil (Cambisol) differing in NOM quality and inorganic ion concentration. We measured the amount of adsorbed organic carbon as well as changes in aromaticity and molecular weight of NOM upon adsorption onto NP. Additionally, the size and zeta potential of NP in both soil solutions were investigated. We observed that the highly hydrophilic NOM of floodplain soil solution rich in inorganic ions strongly adsorbed to Ag but not to TiO2 NP. Instead, sorption to TiO2 NP was observed for the more hydrophobic NOM of the farmland soil with low ionic strength which did not sorb to Ag NP. These differences had a strong effect on NP stability, leading to Ag NP destabilization in case of floodplain soil solution and TiO2 NP stabilization in the presence of farmland soil solution. Our results point out the necessity of studies in more complex systems and suppose that oxic and metallic NP might show very different fate depending on the environment they are exposed to.







Similar content being viewed by others
References
Abraham, P. M., Barnikol, S., Baumann, T., Kuehn, M., Ivleva, N. P., & Schaumann, G. E. (2013). Sorption of silver nanoparticles to environmental and model surfaces. Environmental Science & Technology, 47(10), 5083–5091. https://doi.org/10.1021/es303941e.
Ali, M. A., & Dzombak, D. A. (1996). Competitive sorption of simple organic acids and sulfate on goethite. Environmental Science & Technology, 30(4), 1061–1071. https://doi.org/10.1021/es940723g.
Andersen, D. O., & Gjessing, E. T. (2002). Natural organic matter (NOM) in a limed lake and its tributaries. Water Research, 36(9), 2372–2382. https://doi.org/10.1016/S0043-1354(01)00432-8.
Auffan, M., Rose, J., Bottero, J.-Y., Lowry, G. V., Jolivet, J.-P., & Wiesner, M. R. (2009). Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nature Nanotechnology, 4(10), 634–641. https://doi.org/10.1038/nnano.2009.242.
Baalousha, M., Nur, Y., Römer, I., Tejamaya, M., & Lead, J. R. (2013). Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. Science of the Total Environment, 454–455, 119–131. https://doi.org/10.1016/j.scitotenv.2013.02.093.
Buffle, J., Wilkinson, K. J., Stoll, S., Filella, M., & Zhang, J. (1998). A generalized description of aquatic colloidal interactions: the three-colloidal component approach. Environmental Science & Technology, 32(19), 2887–2899.
Capriel, P., Beck, T., Borchert, H., Gronholz, J., & Zachmann, G. (1995). Hydrophobicity of the organic matter in arable soils. Soil Biology and Biochemistry, 27(11), 1453–1458. https://doi.org/10.1016/0038-0717(95)00068-P.
Celi, L., Schnitzer, M., & Nègre, M. (1997). Analysis of carboxyl groups in soil humic acids by a wet chemical method, Fourier-transform infrared spectrophotometry, and solution-state carbon-13 nuclear magnetic resonance. A comparative study. Soil Science, 162(3), 189–197.
Chowdhury, I., Cwiertny, D. M., & Walker, S. L. (2012). Combined factors influencing the aggregation and deposition of nano-TiO 2 in the presence of humic acid and bacteria. Environmental Science & Technology, 46(13), 6968–6976. https://doi.org/10.1021/es2034747.
Davis, J. A., & Gloor, R. (1981). Adsorption of dissolved organics in lake water by aluminum oxide. Effect of molecular weight. Environmental Science & Technology, 15(10), 1223–1229. https://doi.org/10.1021/es00092a012.
de Laat, A. W. M., & van den Heuvel, G. L. T. (1995). Molecular weight fractionation in the adsorption of polyacrylic acid salts onto BaTiO3. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 98(1), 53–59. https://doi.org/10.1016/0927-7757(95)03096-V.
Ellerbrock, R. H., Höhn, A., & Gerke, H. H. (2001). FT-IR studies on soil organic matter from long-term field experiments. In R. M. Rees, B. C. Ball, C. D. Campbell & C. A (eds) Watson Sustainable Management of Soil Organic Matter (pp. 34–41). Wallingford: CABI Publishing.
Erhayem, M., & Sohn, M. (2014). Effect of humic acid source on humic acid adsorption onto titanium dioxide nanoparticles. Science of the Total Environment, 470–471, 92–98. https://doi.org/10.1016/j.scitotenv.2013.09.063.
French, R. A., Jacobson, A. R., Kim, B., Isley, S. L., Penn, R. L., & Baveye, P. C. (2009). Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environmental Science & Technology, 43(5), 1354–1359. https://doi.org/10.1021/es802628n.
Geelhoed, J. S., Hiemstra, T., & Van Riemsdijk, W. H. (1998). Competitive interaction between phosphate and citrate on goethite. Environmental Science & Technology, 32(14), 2119–2123. https://doi.org/10.1021/es970908y.
Gottschalk, F., Sonderer, T., Scholz, R. W., & Nowack, B. (2009). Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environmental Science & Technology, 43(24), 9216–9222. https://doi.org/10.1021/es9015553.
Gottschalk, F., Sun, T., & Nowack, B. (2013). Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environmental Pollution, 181, 287–300. https://doi.org/10.1016/j.envpol.2013.06.003.
Gu, B., Schmitt, J., Chen, Z., Liang, L., & McCarthy, J. F. (1994). Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environmental Science & Technology, 28(1), 38–46. https://doi.org/10.1021/es00050a007.
Haberhauer, G., & Gerzabek, M. (1999). Drift and transmission FT-IR spectroscopy of forest soils: an approach to determine decomposition processes of forest litter. Vibrational Spectroscopy, 19(2), 413–417. https://doi.org/10.1016/S0924-2031(98)00046-0.
Hur, J., & Schlautman, M. A. (2003). Molecular weight fractionation of humic substances by adsorption onto minerals. Journal of Colloid and Interface Science, 264(2), 313–321. https://doi.org/10.1016/S0021-9797(03)00444-2.
Klitzke, S., Metreveli, G., Peters, A., Schaumann, G. E., & Lang, F. (2015). The fate of silver nanoparticles in soil solution — sorption of solutes and aggregation. Science of the Total Environment, 535, 54–60. https://doi.org/10.1016/j.scitotenv.2014.10.108.
Lecoanet, H. F., Bottero, J.-Y., & Wiesner, M. R. (2004). Laboratory assessment of the mobility of nanomaterials in porous media. Environmental Science & Technology, 38(19), 5164–5169. https://doi.org/10.1021/es0352303.
Li, Y., Yang, C., Guo, X., Dang, Z., Li, X., & Zhang, Q. (2015). Effects of humic acids on the aggregation and sorption of nano-TiO2. Chemosphere, 119, 171–176. https://doi.org/10.1016/j.chemosphere.2014.05.002.
Li, Z., Lowry, G. V., Fan, J., Liu, F., & Chen, J. (2018). High molecular weight components of natural organic matter preferentially adsorb onto nanoscale zero valent iron and magnetite. Science of the Total Environment, 628–629, 177–185. https://doi.org/10.1016/j.scitotenv.2018.02.038.
Louie, S. M., Tilton, R. D., & Lowry, G. V. (2013). Effects of molecular weight distribution and chemical properties of natural organic matter on gold nanoparticle aggregation. Environmental Science & Technology, 47(9), 4245–4254. https://doi.org/10.1021/es400137x.
Louie, S. M., Spielman-Sun, E. R., Small, M. J., Tilton, R. D., & Lowry, G. V. (2015). Correlation of the physicochemical properties of natural organic matter samples from different sources to their effects on gold nanoparticle aggregation in monovalent electrolyte. Environmental Science & Technology, 49(4), 2188–2198. https://doi.org/10.1021/es505003d.
Luo, M., Huang, Y., Zhu, M., Tang, Y., Ren, T., Ren, J., et al. (2016). Properties of different natural organic matter influence the adsorption and aggregation behavior of TiO 2 nanoparticles. Journal of Saudi Chemical Society, 22(2), 146–154. https://doi.org/10.1016/j.jscs.2016.01.007.
Metreveli, G., Frombold, B., Seitz, F., Grün, A., Philippe, A., Rosenfeldt, R. R., et al. (2016). Impact of chemical composition of ecotoxicological test media on the stability and aggregation status of silver nanoparticles. Environmental Science: Nano, 3(2), 418–433. https://doi.org/10.1039/C5EN00152H.
Nason, J. A., McDowell, S. A., & Callahan, T. W. (2012). Effects of natural organic matter type and concentration on the aggregation of citrate-stabilized gold nanoparticles. Journal of Environmental Monitoring, 14(7), 1885. https://doi.org/10.1039/c2em00005a.
Philippe, A., & Schaumann, G. E. (2014). Interactions of dissolved organic matter with natural and engineered inorganic colloids: a review. Environmental Science & Technology, 48(16), 8946–8962. https://doi.org/10.1021/es502342r.
Sänger, A., Reibe, K., Mumme, J., Kaupenjohann, M., Ellmer, F., Roß, C.-L., & Meyer-Aurich, A. (2017). Biochar application to sandy soil: effects of different biochars and N fertilization on crop yields in a 3-year field experiment. Archives of Agronomy and Soil Science, 63(2), 213–229. https://doi.org/10.1080/03650340.2016.1223289.
Sani-Kast, N., Labille, J., Ollivier, P., Slomberg, D., Hungerbühler, K., & Scheringer, M. (2017). A network perspective reveals decreasing material diversity in studies on nanoparticle interactions with dissolved organic matter. Proceedings of the National Academy of Sciences, 114(10), E1756–E1765. https://doi.org/10.1073/pnas.1608106114.
Seijo, M., Ulrich, S., Filella, M., Buffle, J., & Stoll, S. (2009). Modeling the adsorption and coagulation of fulvic acids on colloids by Brownian dynamics simulations. Environmental Science & Technology, 43(19), 7265–7269. https://doi.org/10.1021/es9002394.
Spencer, R. G. M., Bolton, L., & Baker, A. (2007). Freeze/thaw and pH effects on freshwater dissolved organic matter fluorescence and absorbance properties from a number of UK locations. Water Research, 41(13), 2941–2950. https://doi.org/10.1016/j.watres.2007.04.012.
Stankus, D. P., Lohse, S. E., Hutchison, J. E., & Nason, J. A. (2011). Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environmental Science & Technology, 45(8), 3238–3244. https://doi.org/10.1021/es102603p.
Thio, B. J. R., Montes, M. O., Mahmoud, M. A., Lee, D.-W., Zhou, D., & Keller, A. A. (2012). Mobility of capped silver nanoparticles under environmentally relevant conditions. Environmental Science & Technology, 46(13), 6985–6991. https://doi.org/10.1021/es203596w.
Thurman, E. M.. (2012). Organic geochemistry of natural waters (Bd. 2). Springer Science & Business Media.
Thurman, E. M., & Malcolm, R. L. (1981). Preparative isolation of aquatic humic substances. Environmental Science & Technology, 15(4), 463–466. https://doi.org/10.1021/es00086a012.
Van Hoecke, K., De Schamphelaere, K., Van Der Meeren, P., Smagghe, G., & Janssen, C. (2011). Aggregation and ecotoxicity of CeO2 nanoparticles in synthetic and natural waters with variable pH, natural organic matter concentration and ionic strength. Environmental Pollution, 159(4), 970–976. https://doi.org/10.1016/j.envpol.2010.12.010.
Vermeer, A. W. P., & Koopal, L. K. (1998). Adsorption of humic acids to mineral particles. 2. Polydispersity effects with polyelectrolyte adsorption. Langmuir, 14(15), 4210–4216. https://doi.org/10.1021/la970836o.
Vermeer, A., Van Riemsdijk, W., & Koopal, L. (1998). Adsorption of humic acid to mineral particles. 1. Specific and electrostatic interactions. Langmuir, 14(10), 2810–2819.
Weishaar, J. L., Aiken, G. R., Bergamaschi, B. A., Fram, M. S., Fujii, R., & Mopper, K. (2003). Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environmental Science & Technology, 37(20), 4702–4708. https://doi.org/10.1021/es030360x.
Wigger, H., Hackmann, S., Zimmermann, T., Köser, J., Thöming, J., & von Gleich, A. (2015). Influences of use activities and waste management on environmental releases of engineered nanomaterials. Science of the Total Environment, 535, 160–171. https://doi.org/10.1016/j.scitotenv.2015.02.042.
Yang, K., Lin, D., & Xing, B. (2009). Interactions of humic acid with nanosized inorganic oxides. Langmuir, 25(6), 3571–3576. https://doi.org/10.1021/la803701b.
Yin, Y., Shen, M., Tan, Z., Yu, S., Liu, J., & Jiang, G. (2015). Particle coating-dependent interaction of molecular weight fractionated natural organic matter: impacts on the aggregation of silver nanoparticles. Environmental Science & Technology, 49(11), 6581–6589. https://doi.org/10.1021/es5061287.
Acknowledgments
We thank Natascha Volk for her accurate work in the lab realizing many of the batch experiments. Additionally, we want to thank Frederik Büks and George Metreveli for providing the farmland and floodplain soil, respectively. For analyses in the lab, we thank Silke Pabst, Sabine Dumke, and Kotan Yildiz. We are very grateful to Ruth Ellerbrock for the helpful discussions about FT IR spectra.
Funding
The authors acknowledge financial support by the German Research Foundation (DFG) within the research unit FOR 1536 INTERNANO and its subprojects KA 1139/18-2, LA 1398/9 and KL 2909/1-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 75 kb)
Rights and permissions
About this article
Cite this article
Degenkolb, L., Kaupenjohann, M. & Klitzke, S. The Variable Fate of Ag and TiO2 Nanoparticles in Natural Soil Solutions—Sorption of Organic Matter and Nanoparticle Stability. Water Air Soil Pollut 230, 62 (2019). https://doi.org/10.1007/s11270-019-4123-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4123-z