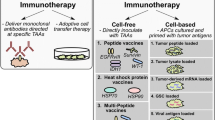
Abstract
Purpose of review
To discuss the current state of glioma vaccine development and highlight the challenges associated with clinical implementation of these approaches.
Recent findings
Vaccination strategies against gliomas have matured considerably during the past years, although proof-of efficacy from controlled clinical trials is still lacking. Advances in antigen discovery, including the definition of neoepitopes including epidermal growth factor receptor variant III (EGFRvIII), isocitrate dehydrogenase (IDH)1R132H and Histone (H)3.3K27M, using multi-omic approaches and computational algorithms allow targeting single antigens, but also implementing truly personalized approaches. In addition, new concepts of vaccine manufacturing including RNA and DNA vaccines improve immunogenicity and applicability in personalized settings.
Summary
As an increasing amount of clinical data defy the concept of the central nervous system (CNS) as a strictly immunoprivileged site, novel vaccine approaches enter the clinic including critical efforts to identify biomarkers of response and resistance and strategies to overcome the immunosuppressive glioma microenvironment.
Similar content being viewed by others
Introduction
Despite considerable advancements in the understanding of the molecular genetics and immunobiology, gliomas remain a major therapeutic challenge. Current therapeutic approaches are limited due to infiltrative growth and the primary and acquired resistance to genotoxic and targeted therapies. Also immunologically, gliomas are regarded as resistant, as “cold” tumors, signified by limited intratumoral immune activation [1]. However, under certain circumstances and with appropriate measures intratumoral immunity can be elicited to become effective. For instance, checkpoint inhibitors—antibodies activating peripheral antitumor immunity—may result in clinical and radiographic response in a minority of glioma patients [2•, 3]. For the majority of glioma patients, however, the paucity of specific antigens is the first limitation for an effective immunotherapy [4]. In general, vaccines aim at inducing or enhancing tumor-specific immune responses similar to antiviral vaccines. Conceptually, however, tumor vaccines differ in that the target is preexisting—hence the term therapeutic vaccine—as opposed to viral vaccines, which are of preventive nature. In addition, tumor vaccines face the general challenge, that it is much more difficult to induce a potent immune response against a tumor, which is immunologically rather perceived as self (or altered self) as opposed to viruses, which are foreign. Finally, glioma vaccines face the specific challenge, that an effective peripheral immune response requires transfer to an immunoprivileged site and overcoming the immunosuppressive microenvironment, which is particularly hostile in glioma [5]. This challenge, however, is not a general obstacle for glioma therapy. Diseases like multiple sclerosis illustrate that T cell immunity against self may be triggered to result in massive—albeit self-limiting—influx of autoantigen-specific T cells into an otherwise healthy CNS with subsequent productive neuroinflammation resulting in the destruction of cells expressing the autoantigen [6]. In addition, expansion of preexisting self-reactive T cells for instance evoked by checkpoint inhibitors may result in deleterious CNS inflammation [7]. Checkpoint inhibitors are blocking antibodies targeting inhibitory cell surface molecules on T cells such as programmed death 1 (PD1) or cytotoxic T lymphocyte-associated protein 4 (CTLA4), thereby resulting in expansion and activation of antigen-specific T cells [8]. While the therapeutic benefit is derived from the expansion and activation of tumor-reactive T cells, common side effects are organ-specific autoimmunity evoked by expansion and activation of autoreactive T cells [7]. Both, preclinical models and patient studies show that checkpoint inhibition alone may be sufficient to cause neuroinflammation affecting meninges or CNS tissue, simply by expanding the peripheral autoreactive T cell pool [9]. Finally, intravenous adoptive transfer of antigen-specific T cells reactive against a tumor antigen and cross-reactive against a CNS antigen may cause massive neuroinflammation in patients [10]. In summary, this highlights that the CNS per se is not a sanctuary site with respect to effective antigen-specific immune response, but is well permissive for the influx and activity of antigen-specific T cells under appropriate circumstances [11]. These circumstances require proper definition. A prerequisite is certainly the sufficient peripheral expansion of antigen-specific T cells with sufficient activity and affinity for the target antigen [12].
Defining appropriate antigens
The therapeutic efficacy of a tumor vaccine critically relies on an appropriate induction and enumeration of antigen-specific T and B cells capable to home to the tumor and exert antitumor activity by killing tumor cells, releasing proinflammatory cytokines and/or producing tumor-specific antibodies. Hence, the identification of appropriate antigens and monitoring of antigen-specific immune responses is critical for assessing efficacy of vaccines [12]. However, since the antigenic profile of tumors in general and gliomas in particular is highly variable and since the appropriate presentation of specific antigens is dependent on highly variable major histocompatibility complex (MHC) allelotypes, the definition of appropriate antigens conceptually requires adequate assessment of the individual patients both with respect to antigenic profile and MHC allelotype. For many years, therapeutic approaches have tried and are—in part—still trying to circumvent this requirement by using autologous whole tumor cell lysates or ribonucleic acids (RNA) as vaccines. Once the process of generating the vaccine under appropriate good manufacturing practice (GMP) standards is achieved, this approach is suitable for all patients, where enough tumor material can be acquired, but remains for the most part ignorant of the specific immune response that is generated. To enhance immunogenicity of tumor lysates or RNA, the material is commonly loaded onto patient autologous dendritic cells (DC) with the aim that these loaded DC, once injected subcutaneously or intradermally—compartments, which provide an environment for efficient T and B cell priming—induce a tumor-specific immune response. Conceptually, this approach comes with two major disadvantages: (1) In order to induce and enumerate a sufficient number of immune cells, antigens must be present in sufficient abundance. As most tumor antigens or tumor-associated antigens are not highly immunogenic (compared with viral antigens), an effective vaccine requires a considerable amount of antigen. For instance, most tumor vaccines utilize between 100 and 500 μg of a given peptide (usually between 10 and 30 amino acids long) for a single vaccine dose. A tumor lysate contains millions of potential antigenic proteins, which individually will be present in most likely insufficient amounts to mount an effective immune response. (2) As tumor cell vaccines are ignorant of the relevant antigens, monitoring of immune responses and correlation to outcome remain vague. Usually, crude assays such as delayed-type hypersensitivity (DTH)—a skin reaction to reinjection of autologous tumor material after vaccination—are employed. Since the relevant antigens, both for the antitumor immune response and the DTH are not defined, it is impossible to take DTH as a firm readout even if one assumes that the antigenicity is overlapping and truly relevant for antitumor immunity. Finally, these early developments are derived from concepts developed in immunogenic, so-called “hot” tumors such as melanoma. As current failures in glioma immunotherapy cannot simply be attributed to the sanctuary tumor location in an immunoprivileged organ, but rather by the profoundly immunosuppressive microenvironment, vaccine approaches to gliomas require considerable refinement to be effective.
Tumor-associated antigens and warehouse approaches
Antigens targeted by glioma vaccines have long been confined to tumor-associated antigens (TAA), which are—from an immunological point of view—self-antigens, that are enriched if not exclusively expressed by tumor cells. Hence, vaccines targeting self-antigens are generally viewed as safe, although autoimmunity to the CNS caused by cross-reactivity may occur [10]. Historically, melanoma antigens also expressed by gliomas, such as MAGE-A1/3, TRP-2, or gp100, have been exploited, as there are very few glioma-specific TAA. Over the years, the panel of self-antigens has been expanded by antigens believed to be enriched in glioma stem cells such as Sox2, HER2, IL13Rα2, and AIM-2. For these antigens, immunodominant epitopes have been defined for the most common MHC class I allelotypes, HLA-A2, and HLA-A24 [13]. The induction of an efficacious antitumor immunity may be hampered on an individual patient level independent of the major class I allelotype and TAA expression by the tumor, for instance by the expression of self-antigens in the thymus, resulting in central T cell tolerance and the development of antigen-specific immunosuppressive T regulatory cells [14]. Therefore, many current clinical trials targeting self-antigens combine multiple epitopes. For regulatory reasons, some approaches even select one or multiple vaccines from a warehouse of vaccines based on individual antigen expression, antigen presentation, and/or preexisting immune responses. ICT-107 is a dendritic cell (DC)-based vaccine comprised of six antigens enriched in glioma stem cells: HER2, TRP-2, gp100, MAGE-A1, IL13Rα2, and AIM-2 [15]. After a randomized, double-blind phase 2 clinical trial showed a slight survival signal in the experimental group particularly in HLA-A2 positive (HLA-A2+) patients [16], a randomized, double-blind, placebo-controlled phase 3 registration trial was initiated in 500 HLA-A2+ patients with newly diagnosed glioblastoma (EORTC1587, NCT02546102). This trial is currently suspended due to insufficient funds provided by the company. Vaccine trials targeting TAA assume that expression in tumors result in recognition by the immune system. Only recently, presentation of TAA in glioblastoma tissue has been addressed experimentally using mass-spectrometry-based HLA ligandome analyses [17•]. Integrating such ligandome analyses into the TAA discovery algorithm consisting of expression analyses and immunogenicity testing helps to significantly enrich the pool of relevant TAA. IMA950 is a warehouse pool of tumor-associated peptides (TUMAP) selected based on HLA ligandome, expression, and immunogenicity analyses, which has demonstrated safety and immunogenicity in a phase 1 clinical trial (NCT01920191) in HLA-A2+ patients with newly diagnosed glioblastoma [18].
All vaccines targeting TAA in glioma suffer from the lack of proof of efficacy from controlled clinical trials. The most important question to be addressed is whether the magnitude and phenotype of a peripheral immune response induced by a vaccine is sufficient to translate into an effective intratumoral antiglioma immunity. The answer to this question clearly requires new treat-biopsy-treat trial concepts. The important impact of the immunosuppressive glioma microenvironment is discussed elsewhere [5].
Targeting non-neoplastic cells
The majority of vaccines target tumor antigens or tumor-associated antigens with the aim at inducing immune responses attacking tumor cells. The tumor-specific nature of non-neoplastic tumor compartments such as the vasculature or stromal cells, however, offer the possibility to target antigens present in the non-neoplastic tumor compartment. The idea is that creating an immune response against tumor-specific non-neoplastic cells contributing to the malignant phenotype of tumors will result in a therapeutic effect. One example is a vaccine targeting the vascular endothelial growth factor receptor type 2 (VEGFR2), which is strongly expressed on proliferating endothelial cells of the glioblastoma vasculature [19]. An oral vaccine (VXM01) consisting of an VEGFR2 expression plasmid encoded in live, attenuated Salmonella bacteria has been developed. After oral ingestion, the antigen is presented in the gut-associated lymphatic tissue following infection with VEGFR2-encoding Salmonella and induces antibody and T cell responses against VEGFR2 [20]. After proof-of-principle studies in pancreatic cancer [21], a phase I clinical trial has been conducted in eight patients with recurrent operable glioblastoma. To allow assessment of intratumoral immune responses, patients with recurrent glioblastoma were included prior to a planned re-resection. The vaccine was initiated and re-resection was postponed to allow for four vaccines prior to re-resection. VXM01 was then continued after resection in four-weekly intervals. VXM01 was safe and resulted in specific peripheral immune responses as well as accumulation of tumor-infiltrating T cells in post-vaccine tumor tissue. Magnetic resonance imaging (MRI) parameters of tumor angiogenesis were affected by the treatment implying vascular normalization and there was one patient with an objective response [22]. A trial combining VXM01 with checkpoint blockade is underway.
Neoantigen-specific vaccines
Neoantigens are tumor antigens, which arise de novo from mutated or variant proteins, oftentimes constituted by a single nucleotide variant. They are exclusively present only in the tumor tissue and not in healthy tissue. With few exceptions, most neoantigens are private antigens as they are derived from patient-specific alterations [14]. Relevant examples of shared neoantigens in gliomas include the variant III of the epidermal growth factor receptor (EGFRvIII), the most common point mutation in the gene for isocitrate dehydrogenase type 1 (IDH1) resulting in the variant protein IDH1R132H, and the most common point mutation in the histone-3 gene H3F3A resulting in the variant protein H3.3K27M. EGVRvIII is generated by alternative splicing of exons 2–7, resulting in a fusion of exons 1 and 8. The peptide sequence spans a neoantigen generated by the fused exons. EGFRvIII is present in 20–30% of glioblastomas and commonly co-expressed with the wild-type variant, even in single tumor cells [23]. A peptide vaccine targeting EGFRvIII conjugates the peptide to the adjuvant keyhole limpet hemocyanin (KLH). The vaccine induces anti-EGFRvIII antibody responses in patients with EGFRvIII-positive tumors [24]. Following non-controlled phase I/II studies [25], a randomized registration trial was conducted (ACT-IV) to test the efficacy of EGFRvIII Pep-KLH (rindopepimut®) combined with standard radiochemotherapy in patients with newly diagnosed EGFRvIII-positive glioblastoma (NCT01480479). Treatment with rindopepimut® did not result in prolongation of overall survival compared with placebo vaccination [26•]. It is unclear, whether the vaccine was not active at all or not active enough as only antibody responses but not T cell responses were reported and as the antibody responses did not correlate with the outcome. The loss of EGFRvIII in about 60% of recurrent tumor tissue, which has initially been thought to be a mechanism of immune escape [27] appears to reflect the natural course of disease progression [28] as it has been observed both in the vaccine and the control arm of the ACT-IV trial with similar frequency. In view of this spontaneous antigen loss the results of the ReACT trial (NCT01498328), which is a placebo-controlled, randomized phase II study in patients with recurrent glioblastoma, where rindopepimut® was given in combination with the antiangiogenic agent bevacizumab, have to be viewed with caution [29]. While the trial demonstrated an increase in overall survival, but not progression-free survival—the primary endpoint—in the experimental arm, the majority of patients were included based on the EGFRvIII status in the primary tumor tissue, while EGFRvIII status at recurrence remained unknown [30]. While there is no evidence of immune evasion, targeting a subclonal antigen, which is not expressed in all cells of a given tumor, such as EGFRvIII, is a conceptual challenge for vaccines, particularly when there is evidence of spontaneous antigen loss due to clonal selection during disease progression [26•]. In contrast, targeting a clonal antigen, which is expressed in all tumor cells, should offer a therapeutic advantage. Clonality of a neoantigen is usually observed with driver mutations, such as IDH1R132H and H3.3K27M.
A peptide vaccine spanning the amino acid exchange at position 132 has been developed after preclinical data demonstrated that (a) the mutated peptide is presented on MHC class II molecules of common allelotypes [31], (b) peptide vaccination induced mutation-specific CD4+ T helper cell responses in MHC-humanized mice sufficient to control the growth of syngeneic IDH1-mutant sarcomas, and (c) spontaneous mutation-specific CD4+ T helper cell and antibody responses can be detected in some patients with IDH1R132H-mutated gliomas [32••]. The development of this vaccine, which does not induce mutation-specific CD8+ cytotoxic T lymphocytes (CTL) [33], supports the relevance of T helper cell responses in tumor immunology [34•] and expands the breadth of targetable antigens. The multicenter first-in-man phase I Neuro-oncology Working Group of the German Cancer Society (NOA-16) trial (NCT02454634) has completed accrual with 33 evaluable patients with newly diagnosed IDH1R132H-expressing high-grade astrocytomas at eight German sites [35]. Patients have received a total of eight vaccines comprised of a 20-mer peptide emulsified in Montanide-ISA51® in addition to combined radiochemotherapy. Mature outcome data are expected in Q2 2018. More recently, H3.3K27M, which is present as a clonal single nucleotide variant (SNV) in the majority of diffuse intrinsic pontine gliomas (DIPG) has been identified as a neoantigen. Unlike IDH1R132H, H3.3K27M comprises a class I and class II-restricted epitope, capable of inducing both CD4+ and CD8+ T cell responses in preclinical models [36]. While a peptide vaccine trial in children with DIPG is currently ongoing (NCT02960230), the cloning of an H3.3K27M-specific T cell receptor recognizing the neoantigen on HLA-A2-positive tumor cells [37•] opens the possibility for adoptive T cell therapy.
Personalized vaccine concepts
The fact that most neoepitopes are private necessitates the generation of personalized vaccines based on the patient-individual neoepitope profile. Neoepitope discovery algorithms are based on whole exome sequencing (WES) followed by a computational pipeline predicting HLA-binding of mutated epitopes [34•]. It is assumed that roughly 1–3% of all non-synonymous mutations result in immunogenic neoepitopes [38]. In glioblastoma, the average number of non-synonymous mutations is 30–50 per megabase, which is at least an order of magnitude lower than that in classic immunogenic tumors such as melanoma [39]. Patient-specific vaccines constitute a regulatory challenge in that approval needs to be obtained for the full process starting from neoepitope discovery to formulation of the patient-specific vaccine(s). Based on a proof-of-concept study in melanoma [40••, 41••], a phase I study in patients with newly diagnosed O6-methylguanine DNA methyltransferase (MGMT)-promoter unmethylated glioblastoma (NeoVax) is ongoing. This study aims at testing feasibility, safety, and immunogenicity of a personalized peptide vaccine (NeoVax) encompassing neoepitopes relevant for the individual patient (NCT02287428). The vaccine is given after completion of radiotherapy. The European Glioma Actively Personalized Vaccine Consortium (GAPVAC), has added another level of complexity in addition to integrating a personalized vaccine into the primary treatment of patients with newly diagnosed glioblastoma. GAPVAC-101 (NCT02149225) is a multicenter phase I feasibility, safety, and immunogenicity trial, for which the selection and production of the personalized peptide vaccine was based not only on WES but also on HLA ligandome analyses providing additional information of the actual presentation of relevant epitopes on HLA molecules in the tumor tissue. While efficacy and immunogenicity data from this trial await reporting, this trial demonstrates that performing complex epitope discovery for generating a personalized vaccine product, that can then be applied within three adjuvant temozolomide cycles, is feasible. Future efforts will have to aim at increasing cost-effectiveness as well as immunogenicity and clinical efficacy of personalized vaccine approaches. In this regard, proof-of-concept studies support the application of RNA as a vaccine to enhance neoepitope-specific immune responses and to facilitate the generation of patient-specific vaccines [42].
Conclusions
Although proof of efficacy from controlled clinical trials is still lacking, vaccine development for glioma immunotherapy has gained a considerable momentum in the past years with an increasing breadth in approaches (Table 1). Recent studies have clearly demonstrated that regulatory challenges can be overcome and complex multi-omic approaches can be integrated into personalized strategies taking the heterogeneity of the disease into account. Also, trial designs have evolved to help answer important translational questions to vaccines. Future efforts will have to be directed towards defining appropriate antigens, maximizing specific immune response, translating peripheral immunity into intratumoral immunity and maintaining an effective antiglioma immunity despite the immunosuppressive glioma microenvironment. These efforts require a constant evolution within scientifically driven clinical trials.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Quail DF, Joyce JA. The Microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–41.
• Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–11. This case report for the first time demonstrates response of a glioblastoma with high mutational load to checkpoint inhibition.
Johanns TM, Miller CA, Dorward IG, Tsien C, Chang E, Perry A, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–6.
Platten M, Bunse L, Wick W, Bunse T. Concepts in glioma immunotherapy. Cancer Immunol Immunother. 2016;65(10):1269–75.
Platten M, Ochs K, Lemke D, Opitz C, Wick W. Microenvironmental clues for glioma immunotherapy. Curr Neurol Neurosci Rep. 2014;14(4):440.
Platten M, Steinman L. Multiple sclerosis: trapped in deadly glue. Nat Med. 2005;11(3):252–3.
Wick W, Hertenstein A, Platten M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 2016;17(12):e529–e41.
Mildenberger I, Bunse L, Ochs K, Platten M. The promises of immunotherapy in gliomas. Curr Opin Neurol. 2017;30(6):650–8.
Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol. 2017;13(12):755–63.
Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–51.
Lanz TV, Becker S, Osswald M, Bittner S, Schuhmann MK, Opitz CA, et al. Protein kinase C beta as a therapeutic target stabilizing blood-brain barrier disruption in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2013;110:14735–40.
Weller M, Roth P, Preusser M, Wick W, Reardon DA, Platten M, et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017;13(6):363–74.
Dutoit V, Migliorini D, Dietrich PY, Walker PR. Immunotherapy of malignant tumors in the brain: how different from other sites? Front Oncol. 2016;6:256.
Platten M, Offringa R. Cancer immunotherapy: exploiting neoepitopes. Cell Res. 2015;25(8):887–8.
Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuno MA, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62(1):125–35.
Wen PY, Reardon DA, Phuphanich S, Aiken R, Landolfi JC, Curry WT, et al. A randomized, double-blind, placebo-controlled phase 2 trial of dendritic cell (DC) vaccination with ICT-107 in newly diagnosed glioblastoma (GBM) patients. J Clin Oncol. 2014;32(5s):suppl; abstr 2005.
• Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135(Pt 4):1042–54. This work demonstrates the use of HLA ligandome analyses for defining relevant glioblastoma antigens.
Rampling R, Peoples S, Mulholland PJ, James A, Al-Salihi O, Twelves CJ, et al. A Cancer Research UK first time in human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016;22(19):4776–85.
Abdollahi A, Lipson KE, Sckell A, Zieher H, Klenke F, Poerschke D, et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res. 2003;63(24):8890–8.
Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8(12):1369–75.
Schmitz-Winnenthal FH, Hohmann N, Niethammer AG, Friedrich T, Lubenau H, Springer M, et al. Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: a randomized, placebo-controlled, phase 1 trial. Oncoimmunology. 2015;4(4):e1001217.
Wick W, Wick A, Nowosielski M, Sahm F, Riehl D, Arzt M, et al. VXM01 phase I study in patients with resectable progression of a glioblastoma. J Clin Oncol. 2017;35:suppl; abstr 2061.
Congdon KL, Gedeon PC, Suryadevara CM, Caruso HG, Cooper LJ, Heimberger AB, et al. Epidermal growth factor receptor and variant III targeted immunotherapy. Neuro-Oncology. 2014;16(Suppl 8):viii20–5.
Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE 2nd, Lally-Goss D, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8(10):2773–9.
Schuster J, Lai RK, Recht LD, Reardon DA, Paleologos NA, Groves MD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro-Oncology. 2015;17(6):854–61.
• Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–85. Albeit negative, this is the first randomized double-blind phase 3 study testing the efficacy of a neoepitope-specific vaccine in glioblastoma.
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–9.
van den Bent MJ, Gao Y, Kerkhof M, Kros JM, Gorlia T, van Zwieten K, et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro-Oncology. 2015;17(7):935–41.
Platten M. EGFRvIII vaccine in glioblastoma-InACT-IVe or not ReACTive enough? Neuro-Oncology. 2017;19(11):1425–6.
Reardon DA, Schuster J, Tran DD, Fink KL, Nabors LB, Li G, et al. ReACT: overall survival from a randomized phase ii study of rindopepimut (CDX-110)plus bevacizumab in relapsed glioblastoma. J Clin Oncol. 2015;33:suppl; abstr 2009.
Bunse L, Schumacher T, Sahm F, Pusch S, Oezen I, Rauschenbach K, et al. Proximity ligation assay evaluates IDH1R132H presentation in gliomas. J Clin Invest. 2015;125(2):593–606.
•• Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–7. This paper identifies a common mutation in gliomas to result in a targetable neoepitope and demonstrates the therapeutic relevance of neoepitope-specific CD4 T cell responses.
Schumacher T, Bunse L, Wick W, Platten M. Mutant IDH1: an immunotherapeutic target in tumors. Oncoimmunology. 2014;3(12):e974392.
• Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–6. This paper defines a workflow for defining neoepitope-specific vaccines and characterizes their mode of action.
Platten M, Schilling D, Bunse T, Sahm F, Hueckelhoven A, Schenkel I, et al. A mutation-specific peptide vaccine targeting IDH1R132H in patients with newly diagnosed malignant astrocytomas: a first-in-man multicenter phase I clinical trial of the German Neurooncology Working Group (NOA-16). J Clin Oncol. 2016;34:suppl; abstr TPS2082.
Ochs K, Ott M, Bunse T, Sahm F, Bunse L, Deumelandt K, et al. K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology. 2017;6(7):e1328340.
• Chheda ZS, Kohanbash G, Okada K, Jahan N, Sidney J, Pecoraro M, et al. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. 2017;215(1):141–57. First description of a neoepitope-specific T cell receptor in gliomas.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74.
Johanns TM, Dunn GP. Applied cancer immunogenomics: leveraging neoantigen discovery in glioblastoma. Cancer J. 2017;23(2):125–30.
••Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–21. Both papers show a clinically applicable and effective workflow for individualized mutation-specific vaccines for the treatment of cancer.
•• Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–6. Both papers show a clinically applicable and effective workflow for individualized mutation-specific vaccines for the treatment of cancer.
Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401.
Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, Kagawa N, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108(5):963–71.
Bloch O, Crane CA, Fuks Y, Kaur R, Aghi MK, Berger MS, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro-Oncology. 2014;16(2):274–9.
Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen—A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol. 2011;29(3):337–44.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dennis Riehl declares that he has no conflict of interest.
Michael Platten: Patents: IDH1 and H3.3 vaccine, Tryptophan metabolites, Methods for Detecting Antigen Presentation; Advisory board - Genentech/Roche, Merck, Bayer, Novartis; Research Support – Bayer, Pfizer; Speaker - Bayer, Merck, Medac, Novartis, Teva, Genentech/Roche; VAXIMM.
Lukas Bunse: Patents: H3.3K27M vaccine, Methods for Detecting Antigen Presentation.
Theresa Bunse: Patents: IDH1 and H3.3K27M vaccines, Methods for Detecting Antigen Presentation.
Katharina Ochs: H3.3 vaccine.
Wolfgang Wick: Patents: IDH1 and H3.3 vaccine, Tryptophan metabolites, APG101 companion diagnostics, Methods for Detecting Antigen Presentation; Research Support – Boehringer Ingelheim, Apogenic, Pfizer, Roche, VAXIMM.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Platten, M., Bunse, L., Riehl, D. et al. Vaccine Strategies in Gliomas. Curr Treat Options Neurol 20, 11 (2018). https://doi.org/10.1007/s11940-018-0498-1
Published:
DOI: https://doi.org/10.1007/s11940-018-0498-1