Abstract
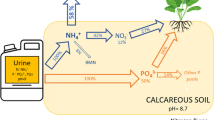
Urine contains about 50 % of the phosphorus (P) and about 90 % of the nitrogen (N) excreted by humans and is therefore an interesting substrate for nutrient recovery. Source-separated urine can be used to precipitate struvite or, through a newly developed technology, nitrified urine fertilizer (NUF). In this study, we prepared 33P radioisotope- and stable 15N isotope-labeled synthetic NUF (SNUF) and struvite using synthetic urine and determined P and N uptake by greenhouse-grown ryegrass (Lolium multiflorum var. Gemini) fertilized with these products. The P and N in the urine-based fertilizers were as readily plant-available in a slightly acidic soil as the P and N in reference mineral fertilizers. The ryegrass crop recovered 26 % of P applied with both urine-based fertilizers and 72 and 75 % of N applied as struvite and SNUF, respectively. Thus, NUF and urine-derived struvite are valuable N and P recycling fertilizers.
Similar content being viewed by others
Introduction
The growing world population and urbanization are resulting in increasing fluxes of phosphorus (P) and nitrogen (N) from agroecosystems to consumers, and eventually into wastewater. Globally, an estimated 3.4 Mt of P are excreted annually by humans, with about 50 % in urine (Mihelcic et al. 2011). If collected and recycled, the P contained in human excreta could satisfy one-fifth of the global P demand (Mihelcic et al. 2011). Nitrogen is mostly excreted in urine (about 90 %; Larsen and Gujer 1996), which could amount to about 28 Mt of human urine N per annum (rough estimate based on an N:P ratio in fresh urine of 16.5 (Udert et al. 2006) and a global annual total amount of urine P of 1.68 Mt (Mihelcic et al. 2011)). Separate collection and treatment of urine have been proposed as an alternative sanitation system that reduces environmental pollution and simplifies nutrient recovery (Larsen et al. 2013). Direct application of human urine as a fertilizer is common practice in many rural areas worldwide. However, this direct recycling pathway cannot be applied in modern cities for several reasons. For example, stored urine has a pungent smell and is prone to N losses due to ammonia volatilization; the high water content renders transport to cropping regions costly; and undesired constituents (e.g., pathogens, micro-pollutants such as synthetic hormones, pharmaceuticals, and their metabolites) pose a health risk to farmers and consumers (Larsen et al. 2013). New technologies are, therefore, needed to recover nutrients in hygienically safe products that can easily be transported to agricultural fields and that are available to plants.
Nearly all the P in urine is present as inorganic phosphate (Ciba-Geigy 1977). Struvite (MgNH4PO4·6H2O) precipitation is a proven technology to recover phosphate from wastewater and has also been applied successfully to recover P from source-separated urine (Etter et al. 2011). If magnesium (Mg) ions are dosed as Mg salts, nearly 100 % of the phosphate can be precipitated (Hug and Udert 2013). However, ammonium recovery in struvite may be only 5 % (Etter et al. 2011) and other valuable nutrients, such as potassium (K), sulfur (S), and micronutrients, are not recovered. A new technology therefore aims at complete nutrient recovery from urine. In a bioreactor, source-separated urine is stabilized by converting volatile ammonia into ammonium nitrate and by mineralizing 90 % of the organic carbon (C). In the second process step, most of the water is removed by distillation (Udert and Wächter 2012). The final product is a concentrated urine solution (nitrified urine fertilizer, NUF). As all the P, K, and S and more than 99 % of the N are recovered, NUF can be considered a multi-elemental fertilizer rich in N (210 mg g−1 dry matter; derived from Udert and Wächter, 2012) with potentially higher market value than struvite.
The state of research regarding P and N uptake by plants from struvite and NUF differs. The P uptake from struvite has been studied in the greenhouse with a variety of test plants on soils covering a range of pH from acidic to slightly alkaline and using synthetic struvite as well as struvite recovered from municipal, agricultural, or industrial wastewaters or from source-separated urine. Despite only 1–5 % of struvite-P being soluble in water (Cabeza et al. 2011; Achat et al. 2014b), all previous studies have found that struvite is as effective as water-soluble reference fertilizers in supplying P to plants (Johnston and Richards 2003; Massey et al. 2009; Antonini et al. 2011; Cabeza et al. 2011). However, apart from Achat et al. (2014b) who used a radioisotope approach in which the soil P was labeled with 33P, but not the fertilizer P, none of these studies differentiated the P taken up by plants into P derived from the soil and P derived from the fertilizer. These studies therefore do not allow accurate estimation of the amount of struvite-P taken up by plants. In addition, the effectiveness of the N contained in struvite has received little attention and simultaneous P and N uptake from struvite has not yet been studied. Moreover, due to its recent development, NUF has not yet been tested as a plant fertilizer.
The aim of the present study was to determine uptake by ryegrass growing in pots in a greenhouse of P and N from 33P- and 15N-labeled struvite (STR) and synthetic-nitrified urine fertilizer (SNUF) produced from synthetic urine as a model for source-separated human urine. The starting hypothesis was that SNUF and STR can supply ryegrass with P and N as effectively as water-soluble mineral P and N fertilizers.
Materials and methods
Labeled synthetic urine-based fertilizer production
The nutrient composition of stored source-separated human urine can be mimicked by synthetic urine (Wilsenach et al. 2007; Tilley et al. 2008), which has been used, e.g., for studying the thermodynamics of struvite precipitation (Ronteltap et al. 2007). Consequently, the effectiveness of human urine P and N can be studied by applying isotope labeling to synthetic urine. In the present study, labeling with 15N and 33P took place during preparation of the synthetic urine solutions.
For STR production, a synthetic urine solution was prepared based on Udert et al. (2006) (Table 1). The biodegradable substrate (80 % of total chemical oxygen demand, COD) was mimicked with acetate. Monosodium phosphate (NaH2PO4·H2O) was first added to deionized water. Thereafter, the equivalent of 27 MBq 33P radioactivity per liter was added in the form of carrier-free 33PO4 3− (33P-phosphoric acid; Hartmann Analytics GmbH, Braunschweig, Germany). After 10 min stirring, all other urine components (Table 1) were dissolved one by one into the 33P-monosodium phosphate solution. The solution was 15N-enriched by adding 15N-ammonium acetate (15NH4C2H3O2, 98 at.% 15N; Sigma Aldrich Chemie GmbH, Buchs, Switzerland) in amounts to achieve 3.2 at.% 15N excess in the solution (excess over the 0.3663 at.% 15N of atmospheric N2) (Table 2). Prior to the precipitation of STR, the pH of the synthetic urine solution was checked (8.9) and Mg (MgCl2) was added at a molar Mg:P ratio of 1.5:1 to ensure that all the P precipitated (Wilsenach et al. 2007). The final solution was stirred for 75 min and then filtered at 500 mbar through cellulose filters (0.45 μm; Whatman, Maidstone, UK). The recovered STR was dried in vacuum desiccators and the STR granules were homogenized into a fine powder with a pestle and mortar and stored in desiccators until use.
The composition of the synthetic-nitrified urine solution used for SNUF production was based on Udert and Wächter (2012). No bicarbonate was added to the synthetic nitrified urine, because the bicarbonate concentration after nitrification is very low. Monosodium phosphate was first dissolved in deionized water, followed by the addition of 27 MBq 33P radioactivity per liter in the form of carrier-free 33PO4 3− (33P-phosphoric acid; Hartmann Analytics GmbH). After 10 min stirring, all other urine components (Table 1) were dissolved one by one into the 33P-monosodium phosphate solution. The nitrified urine solution was spiked with 15N-ammonium nitrate (15NH 154 NO3, 10 at.% 15N; Sigma Aldrich Chemie GmbH) in amounts to achieve 15N enrichment of 3.2 at.% 15N excess in the solution (Table 2). The pH of the synthetic-nitrified urine solution was adjusted with hydrochloric acid (1 M) to match the pH (6) of urine effluent from the nitrification reactor (Udert and Wächter 2012). The distillation of synthetic-nitrified urine was carried out in order to recover a maximum of nutrients in the form of solid SNUF. A volume of 200 mL synthetic-nitrified urine was distilled at once at low pressure using a Büchi Rotavap (for complete distillation protocol, see Udert and Wächter 2012). The solid SNUF was also homogenized into a fine powder and then stored in a vacuum desiccator until application.
Growth experiment
To assess the P and N uptake from SNUF and STR, a pot experiment with Italian ryegrass (Lolium multiflorum var. Gemini) was carried out. The soil used for the growth experiment was taken from the 0–20 cm layer of an arable field in Heitenried (Freiburg, Switzerland), where long-term cropping has been practiced. This acidic soil (pH in water 5.4) was selected because its moderate P availability results in a growth and P uptake response to P fertilizer inputs (Nanzer et al. 2014). It is classified as a Mollic Fluvisol (FAO classification), and has a sandy loam texture (16 % clay, 26 % silt, and 58 % sand), 14 g kg−1 of total C and 1.3 g kg−1 of total N and a low cation exchange capacity (CEC 21 cmol+ kg−1) (Nanzer et al. 2014). Moist soil samples were sieved at 5 mm, then stored until use. The equivalent of 1 kg soil dry matter (DM) was used per pot.
The growth experiment comprised seven treatments (five controls and two urine-based fertilizer treatments) in a completely randomized design with four replicates, resulting in a total of 28 pots (Table 3). Treatments consisted of (1) unfertilized control (0N0P), (2) N-fertilized control (1N0P), (3) P-fertilized control (0N1P), (4 and 5) reference control fertilizers (1N1P and 1N1.5P), and (6 and 7) two urine-based fertilizer treatments (STR and SNUF). For all fertilized control treatments except 1N1.5P, P and N plant uptake were studied using 15N- and 33P-labeled fertilizers (Table 2). The 15N-labeled reference fertilizer was produced using 15NH 154 NO3 (10 at.% 15N; Sigma Aldrich Chemie GmbH), which was mixed with unlabeled NH4NO3 so as to reach the excess of 0.6 at.% 15N (Table 2). The 33P-labeled reference fertilizer was produced by mixing 30 MBq of carrier-free 33PO4 3− (33P-phosphoric acid; Hartmann Analytics GmbH) with KH2PO4 (Table 2). The synthetic urine-based fertilizer treatments consisted of the double 33P- and 15N-labeled STR and double-labeled SNUF treatments. The reference 1P dose was defined at 50 mg P kg−1 soil and the 1N dose at 100 mg N kg−1 soil, based on the plant requirements for intensively managed grassland (Flisch et al. 2009). Assuming a bulk density of 1.3 t m−3 and distribution of the fertilizers within the 0–5 cm soil layer, this would correspond to in kg ha−1, 33 P and 65 N. It was also expected to be within the range of agronomic plant response (Barrow 1985), which was confirmed by response curves (Electronic Supplementary Material, Figs. S1, S2). The urine-based fertilizers were applied on the day of ryegrass sowing. Given its N:P mass ratio, STR with N:P of 1:2.2 supplied the full dose of P (50 mg P kg−1 soil) and adjustments with non 15N-labeled NH4NO3 were made to reach the level of 100 mg N kg−1 soil (Table 3). In contrast, SNUF with N:P of 9.5:1 supplied the entire N dose but needed complementary P in the form of unlabeled KH2PO4 (Table 3). Likewise, the control treatments were P- and N-fertilized on the day of sowing, as indicated in Table 3. For both STR and SNUF, 1N1P was considered the reference (control) treatment. Because of a problem with the N amendment of 1N1P (0.6N added instead of 1N, see footnote of Table 3), 1N1.5P was also used as the control for N. All macronutrients other than P and N were fertilized at rates exceeding 2–3 times the expected plant nutrient uptake. At sowing, all treatments received 200 mg K, 60 mg Ca, 50 mg Mg, and 74 mg S per kg−1 soil DM and micronutrients. In parallel to the main growth experiment, a response curve experiment was carried out in which plant response to graduated amendment levels of P (0–150 mg kg−1 soil DM) and N (0–300 mg kg−1 soil DM) was investigated (Figs. S1, S2). For both the growth experiment and response curve experiment, 1 g of Italian ryegrass seeds was sown per pot. The pots were watered (deionized water) daily up to 50 % of water-holding capacity (560 g kg−1) during the first week, then up to 60 % during the remainder of the growth experiment. The base of the pots was sealed. The growth experiment took place in a greenhouse under controlled climate conditions: 85 % air humidity during the first 2 weeks, then 65 %, photoperiod 12 h day−1, temperature 22°C during day, and 18°C at night and light intensity of maximum ≈500 μmol m−2 s−1 artificial light. Pots were fully randomized three times per week. The ryegrass was harvested 30, 51, and 72 days after sowing. Plants were cut 3 cm above the soil surface at the first and second cut, and at 0.5 cm above the soil surface for the final cut. As the main focus was on P, the growth experiment was designed to provoke a high plant demand for that element and therefore P was not re-applied during the experiment. However, to exclude other nutrient limitations, all treatments were re-fertilized with unlabeled N (NH4NO3), K, Ca, and Mg after the first and second cut, at rates matching those received at the beginning of the experiment. Aboveground plant material was dried for 72 h at 50°C and then finely milled (sieve <2 mm). For each harvest, aboveground plant DM yield, P and N concentrations, and the isotopic composition (at.% 15N and specific activity (SA)) of plant material were determined.
Fertilizer analyses
Total P and N concentrations in STR and SNUF were determined by wet chemical extraction. Subsamples of 25 mg fertilizer DM were dissolved in 100 mL 1 M HCl. Phosphorus concentrations were determined in the diluted extracts by colorimetry (Ohno and Zibilske 1991). Total N in the extract was measured on a TOC/TN analyzer (Skalar FormacsHT/TN, The Netherlands) and nitrate–N and ammonium-N with a continuous flow injection analyzer (Skalar San++ System, The Netherlands). Extracts were prepared in triplicate.
Water-soluble P and N content in STR and SNUF were determined in 1:100 suspensions (2.5 g fertilizer:250 mL deionized water). The suspensions were placed for 30 min on a rotary shaker at 40 rpm, filtered (0.45 µm) through a cellulose filter (Whatman, Maidstone, UK), and soluble P concentrations in diluted filtrates were determined by colorimetry (Ohno and Zibilske 1991). The soluble N concentrations were determined with a TOC–TN analyzer (IL 550 TOC–TN, Hach-Lange, Berlin, Germany). The crystal structure of STR was compared with that of struvite recovered from stored real human urine by X-ray powder diffractometry (XRD) (X’Pert3 Powder, PANalytical, Almelo, The Netherlands). For total N and 15N abundance measurements, SNUF (0.2 mg) and STR (1 mg) subsamples were analyzed on an elemental analyzer (Vario PYRO cube in combustion mode, Elementar, Hanau, Germany) coupled to a mass spectrometer (IsoPrime100, isotope ratio mass spectrometer, Manchester, UK).
Plant analyses
Plant DM samples of 400 mg were ashed for 3 h at 550°C in a muffle oven. The ash was extracted with 2 mL 65 % HNO3 on a hot stirring plate, filtered through a 0.2 µm cellulose acetate syringe filter (Minisart NML, Sartorius, Göttingen, Germany), and P concentration was determined by colorimetry (Ohno and Zibilske 1991). The 33P beta emissions in fertilizer and plant extracts were measured by liquid scintillation counting (TRI-CARB 2500TR Liquid Scintillation Analyzer, Packard Instruments, Meriden, USA). Checks of the measurements revealed no quenching due to matrix effects. The values were corrected for radioactive decay to the day of planting (15 August 2013).
Calculation of P and N uptake from fertilizer
The proportion of plant P originating from fertilizer (Pdff%) can be calculated from the SA in plants grown on soil fertilized with labeled 33P fertilizers (Morel and Fardeau 1990):
where SAplant is the SA (33P/31P, Bq µg−1 P) in plants fertilized with 33P-labeled fertilizer and SAfertilizer is the SA of the respective fertilizer. The amount of fertilizer P taken up by the plants (Pdffmg, mg P kg−1 soil) is
where Puptake is the P taken up by the plants (mg P kg−1 soil), calculated as follows:
where Pconc is the P concentration in shoots (mg P g−1 plant DM) and DM shoot yield is given in g kg−1 soil, which equals the yield per pot. The Pdff% was calculated for each of the three cuts and the resulting Pdff mg values were added together.
The P fertilizer recovery in plants (FertPrec, %) can be derived as follows:
The relative agronomic effectiveness (RAE, %) of STR was calculated by comparing its Pdffmg value to that of the reference fertilizer added at the same fertilization rate (Morel and Fardeau 1990; Nanzer et al. 2014):
For SNUF, the RAE could not be calculated as it was added at a lower dose than with 1N1P. Phosphorus derived from the soil (Pdfs, mg P kg−1 soil) was calculated as follows:
Analogously to P, the proportion of plant N originating from fertilizer (Ndff%, %) was calculated based on isotopic dilution principles (Barraclough 1995):
where 15Nexcess plant and 15Nexcess fertilizer are the 15N enrichments (at.% 15N excess) in the plant and in the fertilizer, respectively. The 15N enrichment of plant samples was calculated by subtracting the 15N abundance in the non N-fertilized plant samples, and the 15N excess of the fertilizers was calculated by subtracting the 15N excess in unlabeled fertilizers.
The total N taken up by plants (Nuptake, mg N kg−1 soil), the amount of N derived from the fertilizer (Ndffmg, mg N kg−1 soil) and from the soil (Ndfs, mg N kg−1 soil), and the fertilizer N recovery in plants (FertNrec, %) were calculated using similar equations as for P (Eqs. 1–4, 6).
The Pdfs and Ndfs values included the P and N contained in the ryegrass seeds. The 1 g seed sown in the pots contained on average 3.1 mg P (Nanzer et al. 2014) and an estimated 20 mg N (Flisch et al. 2009).
Statistical analyses
Statistical analyses were performed with the statistical software package R 2.15.1 (R Core Team 2013). A one-way analysis of variance was run to test the significance of the fertilizer treatments. The Shapiro–Wilk test was performed to test the residuals for normal distribution, followed by a graphical verification by means of quantile–quantile plots. Multiple comparisons were made using the Tukey HSD test (α = 0.01).
Results
Characteristics of processed urine-based fertilizers
All 33P-labeled fertilizers (STR, SNUF, and the KH2PO4 used for the 1P reference water-soluble P fertilizer) had similar SA at the beginning of the pot experiment (Table 2). The urine-based fertilizers had similar 15N enrichment, around 3 at.% 15N, while that in the mineral reference fertilizer was lower. Only small proportions of the total P and N in STR were soluble in water (2–3 % for both), but the P and N contained in SNUF were almost entirely water soluble (Table 2).
Plant dry matter production
Cumulative shoot DM yield of Italian ryegrass varied between 4 and 10 g kg−1 soil. A significant yield response (p < 0.05) to fertilizer treatments was obtained from the first cut onwards and throughout the two remaining cuts (Fig. 1a). The treatments receiving no fertilizer (0N0P) and those receiving either no N or no P (0N1P and 1N0P) produced lower DM yield at the first cut and lower cumulative DM yield than the urine-based fertilizer or 1N1P and 1N1.5P control treatments. The STR and SNUF treatments produced the highest cumulative DM yield, but yield did not differ significantly from that obtained with the 1N1.5P and 1N1P treatments.
Total P and fertilizer P uptake by plants
There was a strong response to water-soluble P fertilizers, as increasing doses resulted in significantly higher plant P uptake values (Fig. S1). Plant P uptake showed a linear response (R 2 = 0.99) to P amendment up to a fertilization rate of 50 mg P kg−1 soil.
All P- and N-fertilized treatments had higher average shoot P concentrations (4.4–5 mg P g−1 DM) than the 1N0P treatment (3.1 mg P g−1 DM). This, together with the higher DM yield, resulted in significantly higher P uptake (Fig. 1b). Total P uptake in the 1N1P, STR, and SNUF treatments was similar (42–45 mg P kg−1 soil). The contribution of fertilizer P (Pdff%) to the total plant P uptake was around 30 % for the 1N1P and STR treatments (Table 4). For SNUF, where less P was added with the labeled fertilizer (Table 3), 5 % of total plant P originated from the SNUF. The three cuts of the ryegrass allowed the availability of fertilizer P to be determined at three time points. It was found that plants fertilized with STR and with reference water-soluble P (1N1P) took up the same amount of fertilizer P (Pdffmg) in all three growth periods. Plants in the STR and 1N1P treatments displayed similar fertilizer P uptake patterns (Fig. 2b). For both treatments, the proportion of fertilizer P in the plant (Pdff%) was higher during the first growing period, while total P uptake increased before the second and third cut (Fig. 2a). The Pdff values for plants in the SNUF treatment remained constant in absolute terms throughout the growth experiment (Pdffmg, Fig. 2b) and relative to total plant P uptake (Pdff%, 5 %), although at a lower level than with 1N1P and STR. The agronomic effectiveness relative to water-soluble P (RAE) of STR was 97 %. In terms of applied fertilizer P recovered in the plants (FertPrec), plants in the SNUF, STR, and 1N1P treatments recovered a similar proportion (26–28 %). The average cumulative Pdfs did not differ between the STR and 1N1P treatments, as the soil delivered an equivalent amount (30 mg P kg−1 soil) to both treatments. The Pdfs for the SNUF treatment could not be assessed, as the soil was fertilized with two P sources (SNUF and KH2PO4).
Total N and fertilizer N uptake by plants
There was a strong response to N, with increased application of water-soluble N resulting in higher plant N uptake (linear response (R 2 = 0.99) up to 150 mg N kg−1 soil; Fig. S2). Thus as for P, the reference fertilization rate of 100 mg N kg−1 soil was in the linear response range.
Nitrogen fertilization resulted in higher N shoot concentrations and hence higher N uptake (Fig. 1c). The shoot N concentration in the 0N1P treatment (14 mg N kg−1) was significantly lower (p < 0.01) than in all N-fertilized treatments (range 28–30 mg N g−1 DM). Nitrogen uptake and Ndff were evaluated primarily for the first cut, as unlabeled NH4NO3 was added after each cut. In the SNUF treatment, at the first cut, over 40 % of plant N taken up originated from the fertilizer (Ndff%) (Table 4). For the 1N1P treatment, fertilizer N accounted for 34 % of plant N uptake (Ndff%), whereas it contributed a smaller proportion in the STR treatment (10 %). The low Ndff% of STR was expected, given the low initial dose (22 mg N kg−1 soil) (Table 3). The fertilizer N recovery pattern was comparable in all three treatments in terms of partial recovery at each cut and total recovery (Table 4). Thus at the first cut, close to 60 % of applied N was recovered in plants (Table 4), at the second cut between 10 and 12 % was recovered, and at the third cut plants recovered between 5 and 7 % of initially applied N. In total, FertNrec ranged between 72 and 77 % for STR, SNUF, and 1N1P (Table 4). The SNUF and 1N1P treatments withdrew similar amounts of N from the soil, resulting in Ndfs values of between 82 and 84 mg N kg−1 soil at the first cut (Table 4). As the STR treatment included two N fertilizers (STR and NH4NO3), Ndfs could not be assessed for that treatment. For all three treatments (1N1P, STR, SNUF), the majority (78–80 %) of total fertilizer N uptake occurred during the first 30 days of growth. The remaining 18–22 % of total fertilizer N uptake took place during the remaining 42 days of the growth experiment (data not shown).
Discussion
Properties of urine-based fertilizers
The water solubility of P and N in STR, which was prepared from artificial urine, was comparable to that of the P and N in struvite precipitated from real human urine (Antonini et al. 2012; Electronic Supplementary Material, Table S1). Likewise, the XRD results suggested similar elemental composition and crystal structures of STR and struvite from real human urine (Electronic Supplementary Material, Fig. S3).
The expected high water solubility of N in SNUF (97 %) was similar to the solubility of N in NUF produced from real human urine (93 % of total N in internal reference NUF), while the P solubility (89 %) of SNUF was higher than the P solubility in the internal reference NUF (57 %) (Table 2, Table S1). The procedure used for SNUF production was based on the chemical composition reported for nitrified human urine (Udert and Wächter 2012), so the differences in P solubility in the final solid NUF products may have been caused by differences in the distillation and drying process used for SNUF and internal reference NUF. Optimization of this process is still in progress and our preliminary findings suggest that it can affect the solubility of P.
Effectiveness of P from urine-based fertilizers
Graduated application of mineral P resulted in increasing plant P uptake (Fig. S1), confirming earlier results obtained on the same soil (Nanzer et al. 2014). The P response curve also showed that the reference dose of 50 mg P kg−1 was in the linear response range, where fertilizer P is used most efficiently. In addition, the linear response in the P amendment treatments ranged from 0 to 50 mg P kg−1 soil, which allowed comparison of plant P recovery (FertPrec) even at the lower 33P-labeled P dose applied with SNUF (8.3 mg P of the total 50 mg P kg−1 soil; Table 3). There was a significantly higher P concentration (p < 0.05) and significantly higher P uptake (p < 0.05) for plants receiving the reference P fertilization dose (1N1P and STR) than plants receiving no P (1N0P) at all three cuts. This indicated that the P fertilizers were plant-available throughout the growth experiment and that P was a growth-limiting factor for the 1N0P treatment. As confirmation, the P concentration (2.2 mg P g−1 DM) at the first cut of the 1N0P treatment was below the critical concentration of 2.5 mg P g−1 DM reported for ryegrass (Bailey et al., 1997). In contrast, the P concentration (3.4–4.2 mg P g−1 DM) exceeded the threshold for all P-fertilized treatments, indicating sufficient P supply for the ryegrass plants.
The similar proportions and amounts of Pdff for STR and the water-soluble P reference fertilizer (1N1P) accumulated over the entire growth experiment (Table 4) indicate the high effectiveness of P contained in STR. The plant availability of P contained in different struvite products has been extensively studied and confirmed to be effective on a wide range of soils (Massey et al. 2009; Cabeza et al. 2011; Antonini et al. 2012). Using 33P isotopic dilution, Achat et al. (2014b) found an effectiveness (RAE) for synthetic struvite of 102–115 % on a slightly acidic soil (pH 6.5) with three cuts of a mixture of ryegrass and fescue and an initial amendment of 50 mg P kg−1 soil, which was similar to our RAE value (97 %; Table 4). Future studies should address the effectiveness of residual P from STR in the soil and compare it with the effectiveness of the residual P from water-soluble reference fertilizers and other recycling products.
Struvite is considered to be a slow-release P fertilizer (Massey et al. 2009; Rahman et al. 2011), given its low solubility in water (Cabeza et al. 2011; Table 2). Its solubility is also affected by pH, as shown, e.g., in a batch experiment with buffered solutions, where the solubility of struvite-P at different pH values (5.9, 7.0, and 8) was less than for triple superphosphate-P (Massey et al. 2009). In the present study, the highest Pdff% value (35 %) for the STR treatment was recorded at the first cut and was equal to that of the water-soluble mineral P, suggesting that P from STR solubilized rapidly in the soil solution. Homogeneous mixing of finely ground STR into the soil, sufficient daily watering (Lunt et al. 1964), and the acidic character of the soil (Babic-Ivancic et al. 2002; Doyle and Parsons 2002) probably had a positive effect on the dissolution rate of the STR crystals. Thus, our results confirm findings by Cabeza et al. (2011) and Achat et al. (2014a) that the effectiveness of recycled P products such as struvite is more accurately indicated by their dissolution in soil than by solubility tests in batch experiments. The use of 33P-labeled struvite provides a means to study the release kinetics and describe the fate of struvite or NUF products in the matrix of soils with differing properties.
Total fertilizer P recovery in ryegrass was similar for the STR, SNUF, and 1N1P treatments (26–28 %; Table 4), indicating that the nutrients in both urine-based fertilizers were as efficiently used by ryegrass as those in the reference fertilizer. The constant uptake rate of P from SNUF shows that the P was plant-available throughout the growth experiment (Fig. 2b). Germer et al. (2011) suggested that the P and K requirement of crops can be partially met by human urine, but no previous study has investigated P uptake from stored human urine in detail. The agronomic effectiveness of the P contained in STR (97 %) and SNUF was greater than that of the P contained in other recycling products obtained from wastewater, such as sewage sludge (RAE 62–78 %; Frossard et al. 1996) and sewage sludge ash (RAE 4–88 %; Nanzer et al. 2014).
Our results highlight the importance of using P radioisotope-labeling techniques to quantify plant P uptake from fertilizer relative to soil. With the conventional difference method, Pdff is calculated as the difference in P uptake between a fertilized and an unfertilized treatment. For the 1N1P and STR treatments in the present study, the Pdffmg values obtained with this approach (derived from P uptake in Fig. 1b and using the 1N0P treatment as the unfertilized control) exceeded those obtained with 33P radioisotope labeling by about 40 %. This discrepancy suggests that these two treatments increased uptake of soil P by the ryegrass. As mentioned previously, P may have been a plant growth-limiting factor in the 1N0P treatment. It is likely that the P-fertilized treatments allowed greater soil exploration by roots (Morel and Fardeau 1989; Achat et al. 2014b) and therefore took up more soil P, since Pdfs were greater for the P-fertilized (Table 4) than the unfertilized treatment (P uptake in 1N0P; Fig. 1b).
Effectiveness of N from urine-based fertilizers
The strong linear response to mineral N amendment (0–150 mg N kg−1 soil; Fig. S2) revealed that the reference level of 100 mg N kg−1 soil applied at sowing to the fertilized treatments was appropriate. The range obtained allowed the plant N recovery of fertilizer (FertNrec) to be compared, including for the lower 15N-labeled N dose contained in struvite (23 mg N kg−1 soil) compared with SNUF or the water-soluble N reference 1N1P (Table 3). The N concentration in the 0N1P treatment suggested that N was a plant growth-limiting element, since the average N concentration at the first cut (24.5 mg g−1) was already below the critical concentration of 28 mg g−1 indicated for ryegrass (Bailey et al. 1997), and decreased to 13 mg g−1 by the third cut. On the other hand, the N concentration in all treatments receiving the equivalent of 1N or higher was always greater than this critical value.
The similar N recovery (FertNrec, Table 4) of STR, SNUF, and the water-soluble mineral N (1N1P) indicates that the two urine-based fertilizers efficiently supplied N to the ryegrass. Field and greenhouse studies comparing plant N uptake from urine-based and mineral-based fertilizers suggest that human urine N could replace mineral N fertilizers (Germer et al. 2011). The N recovery from 15N-labeled urine from monogastrics (Sorensen and Thomsen 2005) and ruminants (Bosshard et al. 2009) is usually reported to be somewhat lower than that from mineral reference fertilizers, a decrease attributed to ammonia losses. The use of 15N-labeled synthetic urine-based fertilizer would enable this to be tested under field conditions.
As found for P, higher Ndff values were obtained for the 1N1P and SNUF treatments with the difference method (derived from N uptake in Fig. 1c using the 0N1P treatment as the unfertilized control) than with the isotope method. Similarly to P, N addition probably increased N uptake from the, soil because N deficiency developed rapidly in the non N-fertilized treatment (0N1P).
Conclusion
The P and N recovery rates and RAE values obtained here showed that the P and N contained in urine-based fertilizers were as readily available to ryegrass growing on a slightly acidic soil as the P and N in water-soluble mineral fertilizers. There is thus technology available to produce effective P and N fertilizers from source-separated human urine. However, further studies are needed on soils with a wider range of chemical properties (e.g., high pH and high P content) and over a range of pedo-climatic conditions in the field. For NUF, further research with real human urine is required to account for the fate of undesired urine compounds, assess the hygiene safety, and determine whether the more complex composition of real urine influences the plant availability of P and N contained in NUF.
References
Achat, D.L., M.L. Daumer, M. Sperandio, A.C. Santellani, and C. Morel. 2014a. Solubility and mobility of phosphorus recycled from dairy effluents and pig manures in incubated soils with different characteristics. Nutrient Cycling in Agroecosystems 99: 1–15.
Achat, D.L., M. Sperandio, M.L. Daumer, A.C. Santellani, L. Prud’Homme, M. Akhtar, and C. Morel. 2014b. Plant-availability of phosphorus recycled from pig manures and dairy effluents as assessed by isotopic labeling techniques. Geoderma 232–234: 24–33.
Antonini, S., S. Paris, T. Eichert, and J. Clemens. 2011. Nitrogen and phosphorus recovery from human urine by struvite precipitation and air stripping in Vietnam. Clean-Soil Air Water 39: 1099–1104.
Antonini, S., M.A. Arias, T. Eichert, and J. Clemens. 2012. Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere 89: 1202–1210.
Babic-Ivancic, V., J. Kontrec, D. Kralj, and L. Brecevic. 2002. Precipitation diagrams of struvite and dissolution kinetics of different struvite morphologies. Croatica Chemica Acta 75: 89–106.
Bailey, J.S., A. Cushnahan, and J.A.M. Beattie. 1997. The diagnosis and recommendation integrated system (DRIS) for diagnosing the nutrient status of grassland swards: II. Model calibration and validation. Plant and Soil 197: 137–147.
Barraclough, D. 1995. N-15 isotope dilution techniques to study soil nitrogen transformations and plant uptake. Fertilizer Research 42: 185–192.
Barrow, N.J. 1985. Comparing the effectiveness of fertilizers. Fertilizer Research 8: 85–90.
Bosshard, C., P. Sørensen, E. Frossard, D. Dubois, P. Mäder, S. Nanzer, and A. Oberson. 2009. Nitrogen use efficiency of 15N-labelled sheep manure and mineral fertiliser applied to microplots in long-term organic and conventional cropping systems. Nutrient Cycling in Agroecosystems 83: 271–287.
Cabeza, R., B. Steingrobe, W. Romer, and N. Claassen. 2011. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutrient Cycling in Agroecosystems 91: 173–184.
Ciba-Geigy. 1977. Wissenschaftliche Tabellen Geigy, Teilband Körperflüssigkeiten (Scientific tables Geigy. Volume body fluids), 8th ed. Basel: Ciba Geigy. (in German).
Doyle, J.D., and S.A. Parsons. 2002. Struvite formation, control and recovery. Water Research 36: 3925–3940.
Etter, B., E. Tilley, R. Khadka, and K.M. Udert. 2011. Low-cost struvite production using source-separated urine in Nepal. Water Research 45: 852–862.
European Parliament. 2003. Verordnung (EG) Nr 2003/2003 des Europäischen Parlaments und des Rates über Düngemittel, 13 Oktober 2003.
Flisch, R., S. Sinaj, R. Charles, and W. Richner. 2009. Grundlagen für die Düngung im Acker-und Futterbau (GRUDAF) 2009. Agrarforschung 16: 1–97. (in German).
Frossard, E., S. Sinaj, L.M. Zhang, and J.L. Morel. 1996. Effect of soil and sludge properties on the fate of sludge phosphorus in soil-plant systems. Soil Science Society of America Journal 60: 1248–1253.
Germer, J., S. Addai, and J. Sauerborn. 2011. Response of grain sorghum to fertilisation with human urine. Field Crops Research 122: 234–241.
Hug, A., and K.M. Udert. 2013. Struvite precipitation from urine with electrochemical magnesium dosage. Water Research 47: 289–299.
Johnston, A.E., and I.R. Richards. 2003. Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use and Management 19: 45–49.
Larsen, T.A., and W. Gujer. 1996. Separate management of anthropogenic nutrient solutions (human urine). Water Science and Technology 34: 87–94.
Larsen, T.A., K.M. Udert, and J. Lienert. 2013. Source separation and decentralization for wastewater management. London: IWA Publishing.
Lunt, O.R., S.B. Clark, and A.M. Kofranek. 1964. Nutrient availability in soil—Availability of minerals from magnesium ammonium phosphates. Journal of Agricultural and Food Chemistry 12: 497–504.
Massey, M.S., J.G. Davis, J.A. Ippolito, and R.E. Sheffield. 2009. Effectiveness of recovered magnesium phosphates as fertilizers in neutral and slightly alkaline soils. Agronomy Journal 101: 323–329.
Mihelcic, J.R., L.M. Fry, and R. Shaw. 2011. Global potential of phosphorus recovery from human urine and feces. Chemosphere 84: 832–839.
Morel, C., and J.C. Fardeau. 1989. Native soil and fresh fertilizer phosphorus uptake as affected by rate of application and P fertilizers. Plant and Soil 115: 123–128.
Morel, C., and J.C. Fardeau. 1990. Agronomical evaluation of phosphate fertilizer as a nutrient source of phosphorus for crops: Isotopic procedure. Fertilizer Research 24: 115–122.
Nanzer, S., A. Oberson, L. Berger, E. Berset, L. Hermann, and E. Frossard. 2014. The plant availability of phosphorus from thermo-chemically treated sewage sludge ashes as studied by 33P labeling techniques. Plant and Soil 377: 439–456.
Ohno, T., and L.M. Zibilske. 1991. Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Science Society of America Journal 55: 892–895.
R core Team. 2013. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Retrieved 3 Nov 2014, from http://www.R-project.org.
Rahman, M.M., Y. Liu, J.H. Kwag, and C. Ra. 2011. Recovery of struvite from animal wastewater and its nutrient leaching loss in soil. Journal of Hazardous Materials 186: 2026–2030.
Ronteltap, M., M. Maurer, and W. Gujer. 2007. Struvite precipitation thermodynamics in source-separated urine. Water Research 41: 977–984.
Sorensen, P., and I.K. Thomsen. 2005. Separation of pig slurry and plant utilization and loss of nitrogen-15-labeled slurry nitrogen. Soil Science Society of America Journal 69: 1644–1651.
Tilley, E., J. Atwater, and D. Mavinic. 2008. Effects of storage on phosphorus recovery from urine. Environmental Technology 29: 807–816.
Udert, K.M., and M. Wächter. 2012. Complete nutrient recovery from source-separated urine by nitrification and distillation. Water Research 46: 453–464.
Udert, K.M., T.A. Larsen, and W. Gujer. 2006. Fate of major compounds in source-separated urine. Water Science and Technology 54: 413–420.
Wilsenach, J.A., C.A.H. Schuurbiers, and M.C.M. van Loosdrecht. 2007. Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Research 41: 458–466.
Acknowledgments
We thank Dr. M. Wächter from Eawag Dübendorf for advice on the production of the urine-based fertilizers; Dr. L. Schönholzer (ETH Zurich) for support in analytical methods; and B. Sinnet (Eawag) for XRD analyses. The experimental work was funded by ETH Zurich. The study was carried out in collaboration with the VUNA project at Eawag, which is funded by the Bill and Melinda Gates Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bonvin, C., Etter, B., Udert, K.M. et al. Plant uptake of phosphorus and nitrogen recycled from synthetic source-separated urine. AMBIO 44 (Suppl 2), 217–227 (2015). https://doi.org/10.1007/s13280-014-0616-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13280-014-0616-6