Abstract
Purpose
Despite curative-intent treatment, recurrence is common for patients with colorectal cancer (CRC). Currently, prediction of disease recurrence and prognostication following surgery is based upon vague clinical factors and more precise and dynamic biomarkers for risk stratification and treatment decisions are urgently needed. Circulating tumor DNA (ctDNA) is a promising biomarker for patients undergoing treatment for resectable CRC.
Methods
In this review, we provide an overview of the data supporting current uses of ctDNA for CRC, including localized CRC and resectable colorectal liver metastases (CLM), as well as descriptions of important ongoing clinical trials using ctDNA in the care of patients with CRC.
Results
The detection of ctDNA following curative-intent therapy is associated with disease recurrence, and multiple trials are investigating its role in determining need and duration for adjuvant therapy for localized CRC. In addition, ctDNA reliably predicts prognosis for patients with CLM, with trials underway studying ctDNA-guided treatment sequencing and intensity.
Conclusion
The detection of ctDNA is a sensitive and dynamic biomarker for disease recurrence in CRC. Many investigations are underway into ctDNA’s potential role in surveillance and treatment algorithms, and it has the potential to become a critical biomarker to determine individualized strategies for treatment sequencing, choice, and duration of therapies.


Similar content being viewed by others
References
Bettegowda C, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24.
Ruibal Morell A. CEA serum levels in non-neoplastic disease. Int J Biol Markers. 1992;7(3):160–6.
Ballehaninna UK, Chamberlain RS. Serum CA 19–9 as a biomarker for pancreatic cancer-a comprehensive review. Indian J Surg Oncol. 2011;2(2):88–100.
Wanebo HJ, et al. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978;299(9):448–51.
Chao M, Gibbs P. Caution is required before recommending routine carcinoembryonic antigen and imaging follow-up for patients with early-stage colon cancer. J Clin Oncol. 2009;27(36):e279–80; author reply e281.a.
Wang Y, et al. Prognostic potential of circulating tumor DNA measurement in postoperative surveillance of nonmetastatic colorectal cancer. JAMA Oncol. 2019;5(8):1118–23.
Diehl F, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci. 2005;102(45):16368–73.
Diehl F, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–90.
Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16(9):398–406.
Dasari A, et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal-Anal Task Forces whitepaper. Nat Rev Clin Oncol. 2020;17(12):757–70.
Murtaza M, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108–12.
Merker JD, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. Arch Pathol Lab Med. 2018;142(10):1242–53.
Reinert T, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–31.
Tie J, et al. Prognostic significance of postsurgery circulating tumor DNA in nonmetastatic colorectal cancer. individual patient pooled analysis of three cohort studies. Int J Cancer. 2020;148(4):1014–26.
Rebuzzi SE, et al. Adjuvant chemotherapy for stage II colon cancer. Cancers. 2020;12(9).
André T, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176–87.
Argilés G, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–305.
Benson AB, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(3):329–359.
Tie J, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346).
Tie J, et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N Engl J Med. 2022;386(24):2261–72.
Taniguchi H, et al. CIRCULATE-Japan: circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 2021;112(7):2915–20.
Miyo M, et al. DENEB: Development of new criteria for curability after local excision of pathological T1 colorectal cancer using liquid biopsy. Cancer Sci. 2022;113(4):1531–4.
Ichimasa K, et al. Risk stratification of T1 colorectal cancer metastasis to lymph nodes: current status and perspective. Gut Liver. 2021;15(6):818–26.
Verbus EA, et al. Circulating tumor DNA as a predictive biomarker in adjuvant chemotherapy for patients with stage 2A colon cancer (COBRA). Ann Surg Oncol. 2021;28(8):4095–7.
Sahin IH, et al. Minimal residual disease-directed adjuvant therapy for patients with early-stage colon cancer: CIRCULATE-US. Oncology (Williston Park). 2022;36(10):604–8.
Anandappa G, et al. TRACC: tracking mutations in cell-free DNA to predict relapse in early colorectal cancer—a randomized study of circulating tumour DNA (ctDNA) guided adjuvant chemotherapy versus standard of care chemotherapy after curative surgery in patients with high risk stage II or stage III colorectal cancer (CRC). J Clin Oncol. 2020;38(15_suppl):TPS4120-TPS4120.
Tie J. Circulating tumour DNA analysis informing adjuvant chemotherapy in stage III colon cancer: a multicentre phase II/III randomised controlled study (DYNAMIC-III). 2017, Australian New Zealand Clinical Trials Registry: ANZCTR.
Schøler LV, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res. 2017;23(18):5437–45.
Renehan AG, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324(7341):813.
Jeffery M, et al. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200.
Snyder RA, et al. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319(20):2104–15.
Nors J, et al. IMPROVE-IT2: implementing noninvasive circulating tumor DNA analysis to optimize the operative and postoperative treatment for patients with colorectal cancer – intervention trial 2 Study protocol. Acta Oncol. 2020;59(3):336–41.
Siegel RL, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Kawaguchi Y, et al. Contour prognostic model for predicting survival after resection of colorectal liver metastases: development and multicentre validation study using largest diameter and number of metastases with RAS mutation status. Br J Surg. 2021;108(8):968–75.
Kanas GP, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301.
Kawaguchi Y, et al. Conditional recurrence-free survival after resection of colorectal liver metastases: persistent deleterious association with RAS and TP53 Co-Mutation. J Am Coll Surg. 2019;229(3):286–294 e1.
Portier G, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24(31):4976–82.
Ychou M, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20(12):1964–70.
Nordlinger B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15.
Bridgewater JA, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(3):398–411.
Narayan RR, et al. Peripheral circulating tumor DNA detection predicts poor outcomes after liver resection for metastatic colorectal cancer. Ann Surg Oncol. 2019;26(6):1824–32.
Kobayashi S, et al. Impact of preoperative circulating tumor DNA status on survival outcomes after hepatectomy for resectable colorectal liver metastases. Ann Surg Oncol. 2021;28(8):4744–55.
Newhook TE, et al. Prospective Study of perioperative circulating tumor DNA dynamics in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2022.
Nishioka Y, et al. Effect of co-mutation of RAS and TP53 on postoperative ctDNA detection and early recurrence after hepatectomy for colorectal liver metastases. J Am Coll Surg. 2022;234(4):474–83.
Reinert T, et al. Circulating tumor DNA for prognosis assessment and postoperative management after curative-intent resection of colorectal liver metastases. Int J Cancer. 2022;150(9):1537–48.
Tie J, et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: A prospective cohort study. PLoS Med. 2021;18(5)–e1003620.
Tie J, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26(8):1715–22.
Author information
Authors and Affiliations
Contributions
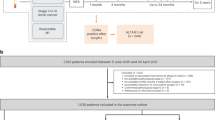
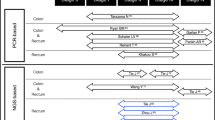
TEN was responsible for the initial conception and outline of the manuscript, and for Figs. 1 and 2. AMA created Table 1. All authors contributed significantly to each draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adams, A.M., Vreeland, T.J. & Newhook, T.E. Circulating Tumor DNA: Towards More Individualized Treatment for Patients with Resectable Colorectal Cancer. J Gastrointest Canc 54, 1071–1081 (2023). https://doi.org/10.1007/s12029-022-00888-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00888-y