Abstract
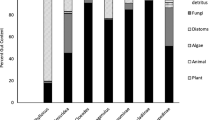
Ephemeral ponds are often dominated by species with both terrestrial and aquatic life phases. Such species have the potential to strongly alter the food web structure of ponds, particularly if they are predators. Here we experimentally tested the effects of salamander larvae (Salamandra salamandra) on invertebrate communities in ephemeral forest ponds. We repeatedly split two ponds into salamander enclosure- and exclosure-segments, and compared the diversity and biomass of potential prey organisms. We used stable isotopes of carbon (δ13C) and nitrogen (δ15N) of resources and consumers to characterise the food web structure. The presence of salamander larvae did not affect abundances of culicid larvae, their preferred prey. The population dynamics of most insect larvae was independent of the presence of salamander larvae, and was instead driven by the timing of hatching and emergence. However, a significant reduction resulting from salamander predation could be detected in the less abundant chironomid larvae. There was no substantial alteration of the food web structure as indicated by stable isotopes. However, the stable isotope results suggest a strong trophic subsidisation from the terrestrial system, which is probably the reason for the weak top-down effects of the salamander larvae on the invertebrate food web.






Similar content being viewed by others
References
Benke, A. C., J. B. W. Smock, A. D. Huryn, L. A. Smock & J. B. Wallace, 1999. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. Journal of the North American Benthological Society 18: 308–343.
Blaustein, L., J. Friedman & T. Fahima, 1996. Larval Salamandra drive temporary pool community dynamics: evidence from an artificial pool experiment. Oikos 76: 392.
Blaustein, J., A. Sadeh & L. Blaustein, 2013. Influence of fire salamander larvae on among-pool distribution of mosquito egg rafts: oviposition habitat selection or egg raft predation? Hydrobiologia 723: 157–165.
Briand, F., 2010. Environmental control of food web structure. Ecology 64: 253–263.
Brodin, T., M. Jonsson, C. Karlsson & F. Johansson, 2007. Intermediate predator impact on consumers weakens with increasing predator diversity in the presence of a top-predator. Acta Oecologica. 31(1): 79–85.
Brooks, R. T., 2000. Annual and seasonal variation and the effects of hydroperiod on benthic macroinvertebrates of seasonal forest (“vernal”) ponds in central Massachusetts, USA. Wetlands 20: 707–715.
Buckley, D., M. Alcobendas, M. García-París & M. H. Wake, 2007. Heterochrony, cannibalism, and the evolution of viviparity in Salamandra salamandra. Evolution Development 9: 105–115.
Caspers, B. A., E. T. Krause, R. Hendrix, M. Kopp, O. Rupp, K. Rosentreter & S. Steinfartz, 2014. The more the better – polyandry and genetic similarity are positively linked to reproductive success in a natural population of terrestrial salamanders (Salamandra salamandra). Molecular Ecology 23: 239–250.
Chesson, P. & N. Huntly, 1997. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. The American Naturalist 150: 519–553.
Crump, M. L., 1979. Intra-population variability in energy parameters of the salamander Plethodon cinereus. Oecologia 247: 235–247.
Davenport, J. M. & D. R. Chalcraft, 2012. Evaluating the effects of trophic complexity on a keystone predator by disassembling a partial intraguild predation food web. The Journal of Animal Ecology 81: 242–250.
Davic, R. D. & H. H. Welsh, 2004. On the ecological roles of salamanders. Annual Review of Ecology Evolution and Systematics Annual Reviews 35: 405–434.
Davies, I. J., 1984. Sampling aquatic insect emergence. In Downing, J. A. & F. H. Rigler (eds.), A Manual on Methods for the Assessment of Secondary Productivity in Freshwaters. Blackwell Scientific Publications, Oxford: 161–221.
Fretwell, S. D., 1987. Food chain dynamics: the central theory of ecology? Oikos 50: 291–301.
Gibbons, J. W., C. T. Winne, D. E. Scott, J. D. Willson, X. Glaudas, K. M. Andrews, B. D. Todd, L. A. Fedewa, L. Wilkinson, R. N. Tsaliagos, S. J. Harper, J. L. Greene, T. D. Tuberville, B. S. Metts, M. E. Dorcas, J. P. Nestor, C. A. Young, T. Akre, R. N. Reed, K. A. Buhlmann, J. Norman, D. A. Croshaw, C. Hagen & B. B. Rothermel, 2006. Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Conservation Biology 20: 1457–1465.
Greig, H. S., S. A. Wissinger & A. R. McIntosh, 2013. Top-down control of prey increases with drying disturbance in ponds: a consequence of non-consumptive interactions? The Journal of animal ecology 82(3): 598–607.
Hocking, D. J., K. J. Babbitt & D. J. Hocking, 2014. Amphibian contributions to ecosystem services. Herpetological Conservation and Biology 9: 1–17.
Holomuzki, J. R., J. P. Collins & P. E. Brunkow, 1994. Trophic control of fishless ponds by tiger salamander larvae. Oikos 71: 55–64.
Katano, O., T. Natsumeda & N. Suguro, 2013. Diurnal bottom feeding of predator fish strengthens trophic cascades to benthic algae in experimental flow-through pools. Ecological Research 28: 907–918.
Leroux, S. J. & M. Loreau, 2008. Subsidy hypothesis and strength of trophic cascades across ecosystems. Ecology letters 11: 1147–1156.
Lincoln, F., 1930. Calculating Waterfowl Abundance on the Basis of Banding Returns. Department of Agriculture, Washington D.C.
Mehner, T., J. Ihlau, H. Do, F. Ho, H. Dörner, F. Hölker, T. Mehner, J. Ihlau & H. Dorner, 2005. Can feeding of fish on terrestrial insects subsidize the nutrient pool of lakes? Limnology and Oceanography 50: 2022–2031.
Nery, T. & D. Schmera, 2016. The effects of top-down and bottom-up controls on macroinvertebrate assemblages in headwater streams. Hydrobiologia 763: 173–181.
O’Neill, B. J. & J. H. Thorp, 2014. Untangling food-web structure in an ephemeral ecosystem. Freshwater Biology 59: 1462–1473.
Paetzold, A., C. J. Schubert & K. Tockner, 2005. Aquatic terrestrial linkages along a braided-river: riparian arthropods feeding on aquatic insects. Ecosystems 8: 748–759.
Peckarsky, B. L., B. L. Kerans, B. W. Taylor & A. R. McIntosh, 2008. Predator effects on prey population dynamics in open systems. Oecologia 156: 431–440.
Peus, F., 1972. Ueber das Subgenus Aedes sensu stricto in Deutschland (Diptera, Culicidae). Zeitschrift fuer angewandte Entomologie 72: 177–194.
Polis, G. A., W. B. Anderson & R. D. Holt, 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28: 289–316.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology Ecological Society of America 83: 703–718.
Reinhardt, T., 2014. New home, new life: the influence of shifts in fire-salamander larval habitat choice on population perfomance and their effect on structure and functioning of pond invertebrate communities. Technische Universität Dresden, Dresden.
Reinhardt, T., A. Paetzold, S. Steinfartz & M. Weitere, 2013. Linking the evolution of habitat choice to ecosystem functioning: direct and indirect effects of pond-reproducing fire salamanders on aquatic-terrestrial subsidies. Oecologia 173: 281–291.
Reinhardt, T., M. Weitere & S. Steinfartz, 2015. Inter-annual weather variability can drive the outcome of predator prey match in ponds. Amphibia-Reptilia 36: 97–109.
Ritchie, E. G. & C. N. Johnson, 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters 12: 982–998.
Rowe, C. L. & W. A. Dunson, 1995. Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of Central Pennsylvania, USA. Oecologia 102: 397–403.
Rubbo, M. J., J. J. Cole & J. M. Kiesecker, 2006. Terrestrial subsidies of organic carbon support net ecosystem production in temporary forest ponds: evidence from an ecosystem experiment. Ecosystems 9: 1170–1176.
Rubbo, M. J., Æ. L. K. Belden & Æ. J. M. Kiesecker, 2008. Differential responses of aquatic consumers to variations in leaf-litter inputs. Hydrobiologia Springer 605: 37–44.
Sabo, J. L. & M. E. Power, 2002. Numerical response of lizards to aquatic insects and short-term consequences for terrestrial prey. Ecology 83: 3023.
Schmitz, O. J. & K. B. Suttle, 2001. Effects of top predator species on direct and indirect interactions in a food web. Ecology 82: 2072–2081.
Schriever, T. A. & D. D. Williams, 2013. Influence of pond hydroperiod, size, and community richness on food-chain length. Freshwater Science 32: 964–975.
Steinfartz, S., M. Veith & D. Tautz, 2000. Mitochondrial sequence analysis of Salamandra taxa suggests old splits of major lineages and postglacial recolonizations of central Europe from distinct source populations of Salamandra salamandra. Molecular Ecology Wiley Online Library 9: 397–410.
Tanentzap, A. J., B. W. Kielstra, G. M. Wilkinson, M. Berggren, N. Craig, P. A. del Giorgio, J. Grey, J. M. Gunn, S. E. Jones, J. Karlsson, C. T. Solomon & M. L. Pace, 2017. Terrestrial support of lake food webs: synthesis reveals controls over cross-ecosystem resource use. Science Advances 3: e1601765.
Thiesmeier, B., 2004. Der Feuersalamander. Laurenti Verlag, Bielefeld.
Walls, S. C. & M. G. Williams, 2001. The effect of community composition on persistence of prey with their predators in an assemblage of pond-breeding amphibians. Oecologia 128: 134–141.
Weitere, M., D. Tautz, D. Neumann & S. Steinfartz, 2004. Adaptive divergence vs. environmental plasticity: tracing local genetic adaptation of metamorphosis traits in salamanders. Molecular ecology 13: 1665–1677.
Wilbur, H. M., 1997. Experimental ecology of food webs: Complex systems in temporary ponds. Ecology 78(8): 2279–2302.
Williams, D. D., 1987. The Ecology of Temporary Waters. Springer, Portland.
Williams, D. D., 1997. Temporary ponds and their invertebrate communities. Aquatic Conservation 7: 105–117.
Zanden, M. J. & J. B. Rasmussen, 1999. Primary consumer δ 13 C and δ 15 N and the trophic position of aquatic consumers. Ecology Ecological Society of America 80: 1395–1404.
Acknowledgements
The study was supported by a scholarship of the Deutsche Bundesstiftung Umwelt (DBU) to TR. This research was possible with the kind permission of the governmental forestry office in Bonn and the Nature Reserve Authorities Bonn who granted the necessary permission for access and sampling of salamander larvae and invertebrates. Also we wish to thank the stable isotope lab of the University of Koblenz- Landau for processing the samples. Furthermore, we thank Amy MacLeod and Sam Armstrong for linguistic improvement of the manuscript and Heidrun Windisch and Patrick Fink for fruitful discussions and support on statistical analyses.
Author contributions
TR, MW, and SS originally developed the project idea and wrote the manuscript. TR conducted fieldwork and data analysis under the supervision of MW and SS. MB supervised the sampling, analyses, and interpretation of the stable isotope samples and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Rights and permissions
About this article
Cite this article
Reinhardt, T., Brauns, M., Steinfartz, S. et al. Effects of salamander larvae on food webs in highly subsidised ephemeral ponds. Hydrobiologia 799, 37–48 (2017). https://doi.org/10.1007/s10750-017-3195-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3195-2