Abstract
Objectives
To characterize testosterone profile changes over time in a cohort of prostate cancer (PCa) patients managed with active surveillance (AS) and to assess its correlation with the initial disease characteristics and further progression.
Methods
We conducted retrospective chart review of PCa patients managed with AS. Patients were followed with PSA, total, free and bioavailable testosterone measurements, physical examination, and by repeat biopsies or periodic magnetic resonance imaging. Disease progression was identified by follow-up biopsy changes or by imaging. A Cox proportional hazard regression models were used to assess the association between testosterone profile at baseline and the risk of progression.
Results
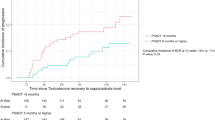
For the 122 patients included in analyses, the mean age at diagnosis was 65.8 years; the mean follow-up time was 7.8 years. At baseline, 108 (88.5%) patients had a Gleason score of ≤ 6. In all, 45 (36.8%) patients had disease progression, with a mean time to progression of 4.6 years. During follow-up, PSA levels showed a rising trend, while testosterone profile levels showed a trend of decrease over time. There was no significant correlation between PSA and testosterone profile (total, free, and bioavailable) level changes over time (ρ = − 0.14, − 0.11 and − 0.16, P = 0.16, 0.34, and 0.20, respectively). In addition, multivariable analysis showed that serum-free testosterone was an independent predictor of disease progression (HR 0.93, 95% CI 0.88–0.99, P = 0.029).
Conclusion
Our study results showed that testosterone profile measurements tended to decrease over time in PCa patients managed with AS. Free testosterone was a significant independent variable of disease progression.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Canadian Cancer Society’s Advisory Committee on Cancer Statistics (2016) Canadian cancer statistics 2016. Canadian Cancer Society, Toronto
Singer EA, Kaushal A, Turkbey B, Couvillon A, Pinto PA, Parnes HL (2012) Active surveillance for prostate cancer: past, present and future. Curr Opin Oncol 24(3):243–250
Dall’Era MA, Klotz L (2017) Active surveillance for intermediate-risk prostate cancer. Prostate Cancer Prostatic Dis 20(1):1–6
Hayes JH, Ollendorf DA, Pearson SD, Barry MJ, Kantoff PW, Stewart ST et al (2010) Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA 304(21):2373–2380
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S et al (2014) Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 33(3):272–277
Dall’Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR et al (2012) Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 62(6):976–983
San Francisco IF, Rojas PA, DeWolf WC, Morgentaler A (2014) Low free testosterone levels predict disease reclassification in men with prostate cancer undergoing active surveillance. BJU Int 114(2):229–235
Loeb S, Bruinsma SM, Nicholson J, Briganti A, Pickles T, Kakehi Y et al (2015) Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol 67(4):619–626
Gurbuz C, Canat L, Atis G, Guner B, Caskurlu T (2012) The role of serum testosterone to prostate-specific antigen ratio as a predictor of prostate cancer risk. Kaohsiung J Med Sci 28(12):649–653
Mearini L, Zucchi A, Nunzi E, Villirillo T, Bini V, Porena M (2013) Low serum testosterone levels are predictive of prostate cancer. World J Urol 31(2):247–252
Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84(10):3666–3672
Albisinni S, De Nunzio C, Tubaro A, Barry WT, Banez LL, Freedland SJ (2012) Greater percent-free testosterone is associated with high-grade prostate cancer in men undergoing prostate biopsy. Urology 80(1):162–167
Dai B, Qu Y, Kong Y, Ye D, Yao X, Zhang S et al (2012) Low pretreatment serum total testosterone is associated with a high incidence of Gleason score 8–10 disease in prostatectomy specimens: data from ethnic Chinese patients with localized prostate cancer. BJU Int 110(11 Pt B):E667–E672
Gao Y, Jiang CY, Mao SK, Cui D, Hao KY, Zhao W et al (2016) Low serum testosterone predicts upgrading and upstaging of prostate cancer after radical prostatectomy. Asian J Androl 18(4):639–643
Leon P, Seisen T, Cussenot O, Drouin SJ, Cattarino S, Comperat E et al (2015) Low circulating free and bioavailable testosterone levels as predictors of high-grade tumors in patients undergoing radical prostatectomy for localized prostate cancer. Urol Oncol 33(9):384.e21–384.e27
Hellstrom WJ, Paduch D, Donatucci CF (2012) Importance of hypogonadism and testosterone replacement therapy in current urologic practice: a review. Int Urol Nephrol 44(1):61–70
Petak SM, Nankin H, Spark R, Swerdloff R, Rodriguez-Rigau L (2002) American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 8(6):440–456
Lunenfeld B, Mskhalaya G, Zitzmann M, Arver S, Kalinchenko S, Tishova Y et al (2015) Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 18(1):5–15
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86(2):724–731
Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD et al (2010) Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 363(2):123–135
Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C (2006) Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60(7):762–769
Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE et al (2007) Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 92(11):4241–4247
Cooper CS, Perry PJ, Sparks AE, MacIndoe JH, Yates WR, Williams RD (1998) Effect of exogenous testosterone on prostate volume, serum and semen prostate specific antigen levels in healthy young men. J Urol 159(2):441–443
Roddam AW, Allen NE, Appleby P, Key TJ (2008) Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 100(3):170–183
Muller RL, Gerber L, Moreira DM, Andriole G, Castro-Santamaria R, Freedland SJ (2012) Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the Reduction by Dutasteride of Prostate Cancer Events trial. Eur Urol 62(5):757–764
Morgentaler A (2006) Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol 50(5):935–939
Morgentaler A (2012) Low testosterone levels are related to poor prognosis factors in men with prostate cancer prior to treatment. BJU Int 110(11 Pt B):E547
Morgentaler A, Traish AM (2009) Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 55(2):310–320
Ferro M, Lucarelli G, Bruzzese D, Di Lorenzo G, Perdona S, Autorino R et al (2017) Low serum total testosterone level as a predictor of upstaging and upgrading in low-risk prostate cancer patients meeting the inclusion criteria for active surveillance. OncoTarget 8(11):18424–18434
Author information
Authors and Affiliations
Contributions
Zakaria AS: project development, data collection, data analysis, and manuscript writing. Dragomir A: project development, data analysis, and manuscript writing. Kassouf W: project development and manuscript writing. Tanguay S: project development and manuscript writing. Aprikian A: project development, data analysis, and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required (retrospective study).
Rights and permissions
About this article
Cite this article
Zakaria, A.S., Dragomir, A., Kassouf, W. et al. Changes in the levels of testosterone profile over time in relation to clinical parameters in a cohort of patients with prostate cancer managed by active surveillance. World J Urol 36, 1209–1217 (2018). https://doi.org/10.1007/s00345-018-2270-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2270-2